Matthew T. Bigelow
for the Alzheimer's Disease Neuroimaging Initiative
Artificial Intelligence to Assist in Exclusion of Coronary Atherosclerosis during CCTA Evaluation of Chest-Pain in the Emergency Department: Preparing an Application for Real-World Use
Aug 10, 2020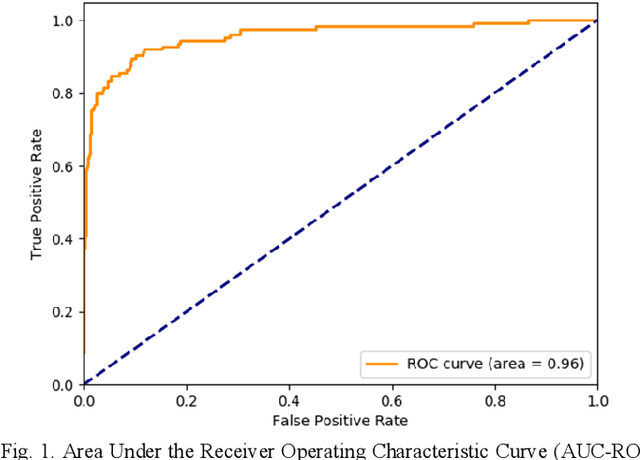
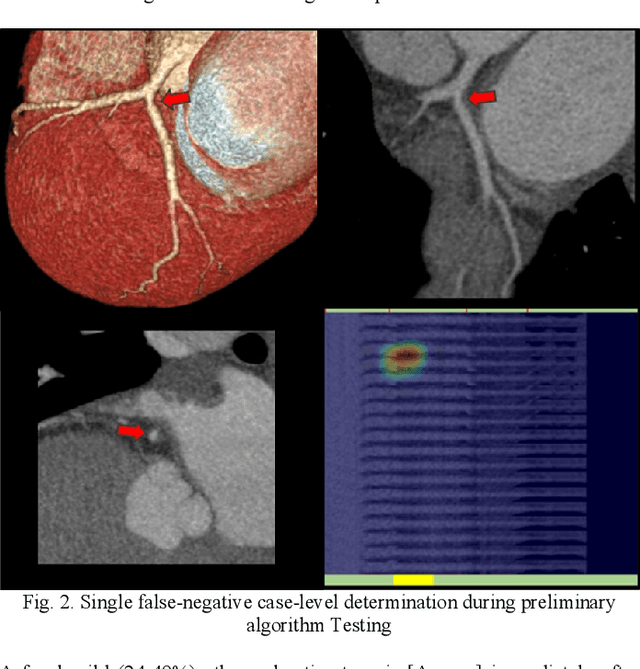
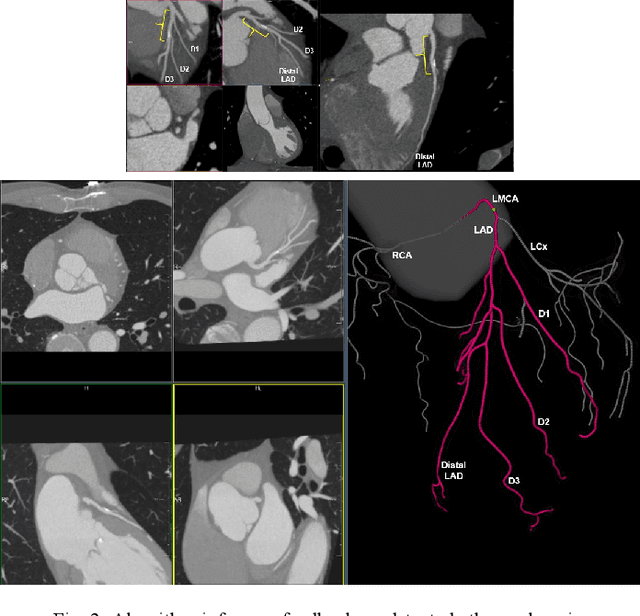
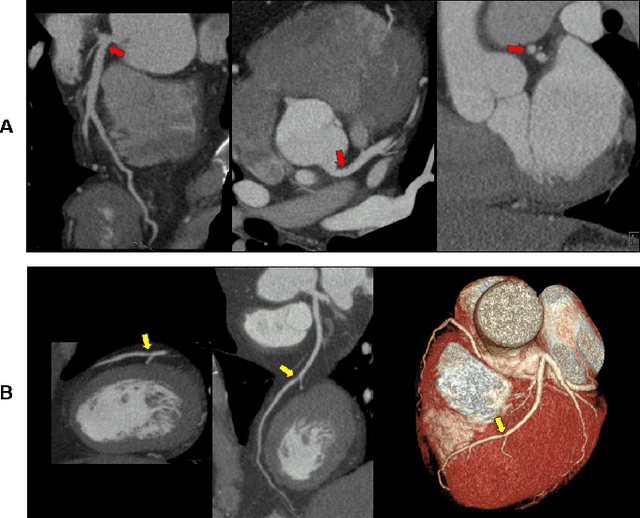
Abstract:Coronary Computed Tomography Angiography (CCTA) evaluation of chest-pain patients in an Emergency Department (ED) is considered appropriate. While a negative CCTA interpretation supports direct patient discharge from an ED, labor-intensive analyses are required, with accuracy in jeopardy from distractions. We describe the development of an Artificial Intelligence (AI) algorithm and workflow for assisting interpreting physicians in CCTA screening for the absence of coronary atherosclerosis. The two-phase approach consisted of (1) Phase 1 - focused on the development and preliminary testing of an algorithm for vessel-centerline extraction classification in a balanced study population (n = 500 with 50% disease prevalence) derived by retrospective random case selection; and (2) Phase 2 - concerned with simulated-clinical Trialing of the developed algorithm on a per-case basis in a more real-world study population (n = 100 with 28% disease prevalence) from an ED chest-pain series. This allowed pre-deployment evaluation of the AI-based CCTA screening application which provides a vessel-by-vessel graphic display of algorithm inference results integrated into a clinically capable viewer. Algorithm performance evaluation used Area Under the Receiver-Operating-Characteristic Curve (AUC-ROC); confusion matrices reflected ground-truth vs AI determinations. The vessel-based algorithm demonstrated strong performance with AUC-ROC = 0.96. In both Phase 1 and Phase 2, independent of disease prevalence differences, negative predictive values at the case level were very high at 95%. The rate of completion of the algorithm workflow process (96% with inference results in 55-80 seconds) in Phase 2 depended on adequate image quality. There is potential for this AI application to assist in CCTA interpretation to help extricate atherosclerosis from chest-pain presentations.
Predicting Rate of Cognitive Decline at Baseline Using a Deep Neural Network with Multidata Analysis
Feb 24, 2020



Abstract:This study investigates whether a machine-learning-based system can predict the rate of cognitive-decline in mildly cognitively impaired (MCI) patients by processing only the clinical and imaging data collected at the initial visit. We build a predictive model based on a supervised hybrid neural network utilizing a 3-Dimensional Convolutional Neural Network to perform volume analysis of Magnetic Resonance Imaging (MRI) and integration of non-imaging clinical data at the fully connected layer of the architecture. The analysis is performed on the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset. Experimental results confirm that there is a correlation between cognitive decline and the data obtained at the first visit. The system achieved an area under the receiver operator curve (AUC) of 66.6% for cognitive decline class prediction.
Are Quantitative Features of Lung Nodules Reproducible at Different CT Acquisition and Reconstruction Parameters?
Aug 14, 2019



Abstract:Consistency and duplicability in Computed Tomography (CT) output is essential to quantitative imaging for lung cancer detection and monitoring. This study of CT-detected lung nodules investigated the reproducibility of volume-, density-, and texture-based features (outcome variables) over routine ranges of radiation-dose, reconstruction kernel, and slice thickness. CT raw data of 23 nodules were reconstructed using 320 acquisition/reconstruction conditions (combinations of 4 doses, 10 kernels, and 8 thicknesses). Scans at 12.5%, 25%, and 50% of protocol dose were simulated; reduced-dose and full-dose data were reconstructed using conventional filtered back-projection and iterative-reconstruction kernels at a range of thicknesses (0.6-5.0 mm). Full-dose/B50f kernel reconstructions underwent expert segmentation for reference Region-Of-Interest (ROI) and nodule volume per thickness; each ROI was applied to 40 corresponding images (combinations of 4 doses and 10 kernels). Typical texture analysis metrics (including 5 histogram features, 13 Gray Level Co-occurrence Matrix, 5 Run Length Matrix, 2 Neighboring Gray-Level Dependence Matrix, and 2 Neighborhood Gray-Tone Difference Matrix) were computed per ROI. Reconstruction conditions resulting in no significant change in volume, density, or texture metrics were identified as "compatible pairs" for a given outcome variable. Our results indicate that as thickness increases, volumetric reproducibility decreases, while reproducibility of histogram- and texture-based features across different acquisition and reconstruction parameters improves. In order to achieve concomitant reproducibility of volumetric and radiomic results across studies, balanced standardization of the imaging acquisition parameters is required.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge