Mario Ries
MRI-Guided High Intensity Focused Ultrasound of Liver and Kidney
Nov 21, 2020



Abstract:High Intensity Focused Ultrasound (HIFU) can be used to achieve a local temperature increase deep inside the human body in a non-invasive way. MRI guidance of the procedure allows in situ target definition. In addition, MRI can be used to provide continuous temperature mapping during HIFU for spatial and temporal control of the heating procedure and prediction of the final lesion based on the received thermal dose. Temperature mapping of mobile organs as kidney and liver is challenging, as well as real-time processing methods for feedback control of the HIFU procedure. In this paper, recent technological advances are reviewed in MR temperature mapping of these organs, in motion compensation of the HIFU beam, in intercostal HIFU sonication, and in volumetric ablation and feedback control strategies. Recent pre-clinical studies have demonstrated the feasibility of each of these novel methods. The perspectives to translate those advances into the clinic are addressed. It can be concluded that MR guided HIFU for ablation in liver and kidney appears feasible but requires further work on integration of technologically advanced methods.
Deep correction of breathing-related artifacts in MR-thermometry
Nov 10, 2020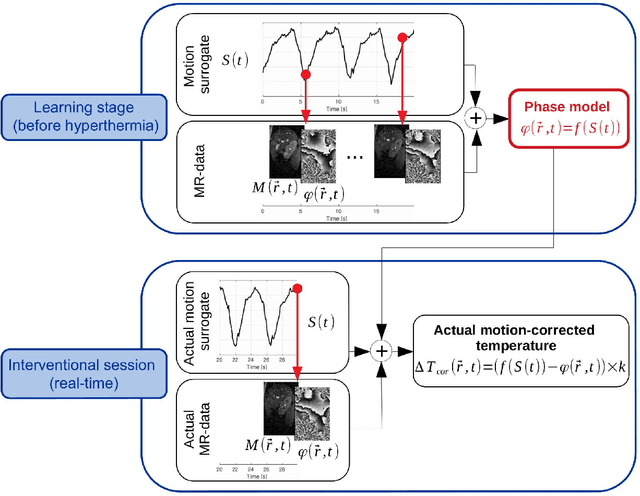
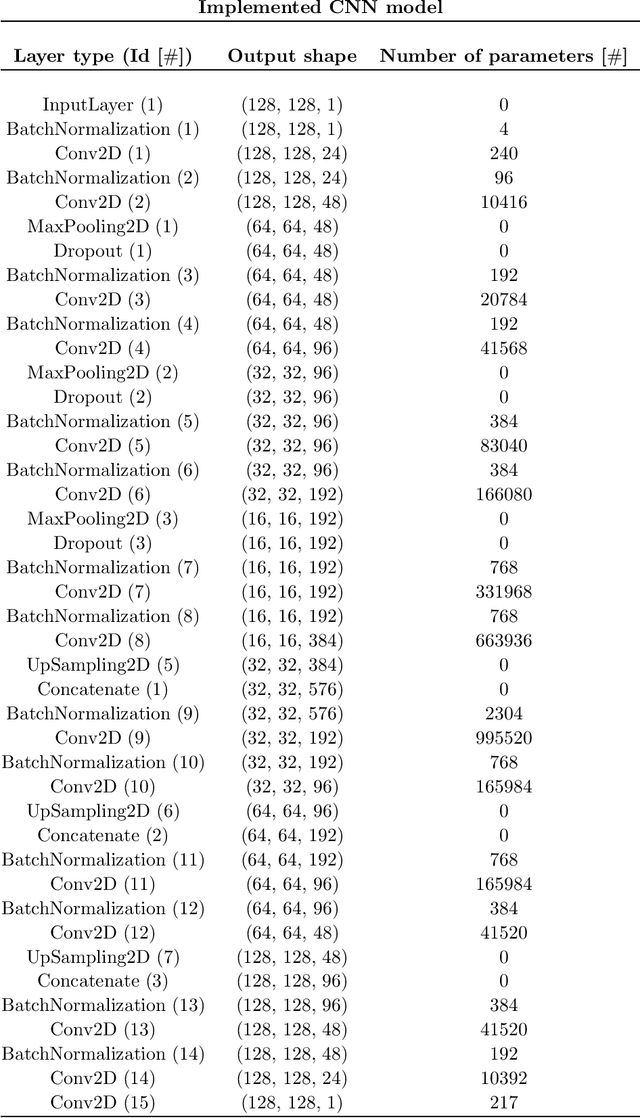
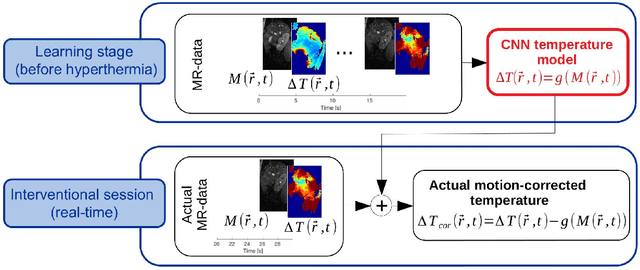
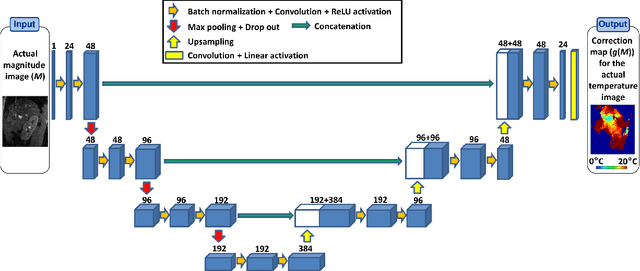
Abstract:Real-time MR-imaging has been clinically adapted for monitoring thermal therapies since it can provide on-the-fly temperature maps simultaneously with anatomical information. However, proton resonance frequency based thermometry of moving targets remains challenging since temperature artifacts are induced by the respiratory as well as physiological motion. If left uncorrected, these artifacts lead to severe errors in temperature estimates and impair therapy guidance. In this study, we evaluated deep learning for on-line correction of motion related errors in abdominal MR-thermometry. For this, a convolutional neural network (CNN) was designed to learn the apparent temperature perturbation from images acquired during a preparative learning stage prior to hyperthermia. The input of the designed CNN is the most recent magnitude image and no surrogate of motion is needed. During the subsequent hyperthermia procedure, the recent magnitude image is used as an input for the CNN-model in order to generate an on-line correction for the current temperature map. The method's artifact suppression performance was evaluated on 12 free breathing volunteers and was found robust and artifact-free in all examined cases. Furthermore, thermometric precision and accuracy was assessed for in vivo ablation using high intensity focused ultrasound. All calculations involved at the different stages of the proposed workflow were designed to be compatible with the clinical time constraints of a therapeutic procedure.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge