Krzysztof Kotowski
KP Labs
Fake or Real: The Impostor Hunt in Texts for Space Operations
Jul 17, 2025Abstract:The "Fake or Real" competition hosted on Kaggle (\href{https://www.kaggle.com/competitions/fake-or-real-the-impostor-hunt}{https://www.kaggle.com/competitions/fake-or-real-the-impostor-hunt}) is the second part of a series of follow-up competitions and hackathons related to the "Assurance for Space Domain AI Applications" project funded by the European Space Agency (\href{https://assurance-ai.space-codev.org/}{https://assurance-ai.space-codev.org/}). The competition idea is based on two real-life AI security threats identified within the project -- data poisoning and overreliance in Large Language Models. The task is to distinguish between the proper output from LLM and the output generated under malicious modification of the LLM. As this problem was not extensively researched, participants are required to develop new techniques to address this issue or adjust already existing ones to this problem's statement.
MASCOTS: Model-Agnostic Symbolic COunterfactual explanations for Time Series
Mar 28, 2025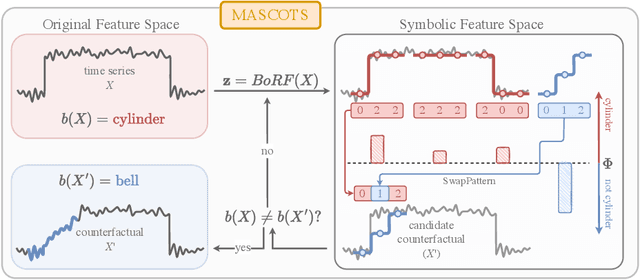
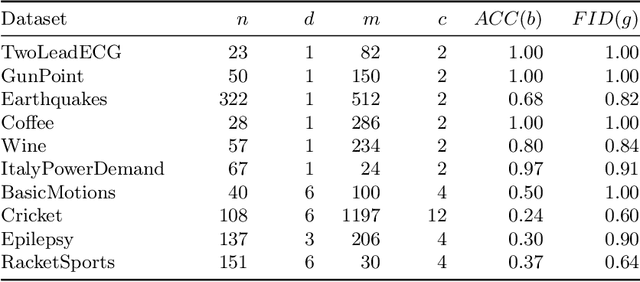
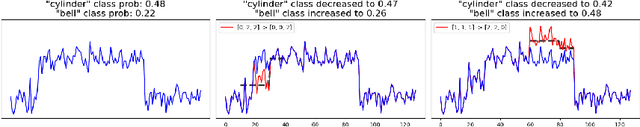
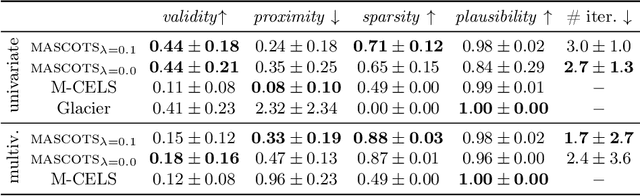
Abstract:Counterfactual explanations provide an intuitive way to understand model decisions by identifying minimal changes required to alter an outcome. However, applying counterfactual methods to time series models remains challenging due to temporal dependencies, high dimensionality, and the lack of an intuitive human-interpretable representation. We introduce MASCOTS, a method that leverages the Bag-of-Receptive-Fields representation alongside symbolic transformations inspired by Symbolic Aggregate Approximation. By operating in a symbolic feature space, it enhances interpretability while preserving fidelity to the original data and model. Unlike existing approaches that either depend on model structure or autoencoder-based sampling, MASCOTS directly generates meaningful and diverse counterfactual observations in a model-agnostic manner, operating on both univariate and multivariate data. We evaluate MASCOTS on univariate and multivariate benchmark datasets, demonstrating comparable validity, proximity, and plausibility to state-of-the-art methods, while significantly improving interpretability and sparsity. Its symbolic nature allows for explanations that can be expressed visually, in natural language, or through semantic representations, making counterfactual reasoning more accessible and actionable.
The OPS-SAT benchmark for detecting anomalies in satellite telemetry
Jun 29, 2024Abstract:Detecting anomalous events in satellite telemetry is a critical task in space operations. This task, however, is extremely time-consuming, error-prone and human dependent, thus automated data-driven anomaly detection algorithms have been emerging at a steady pace. However, there are no publicly available datasets of real satellite telemetry accompanied with the ground-truth annotations that could be used to train and verify anomaly detection supervised models. In this article, we address this research gap and introduce the AI-ready benchmark dataset (OPSSAT-AD) containing the telemetry data acquired on board OPS-SAT -- a CubeSat mission which has been operated by the European Space Agency which has come to an end during the night of 22--23 May 2024 (CEST). The dataset is accompanied with the baseline results obtained using 30 supervised and unsupervised classic and deep machine learning algorithms for anomaly detection. They were trained and validated using the training-test dataset split introduced in this work, and we present a suggested set of quality metrics which should be always calculated to confront the new algorithms for anomaly detection while exploiting OPSSAT-AD. We believe that this work may become an important step toward building a fair, reproducible and objective validation procedure that can be used to quantify the capabilities of the emerging anomaly detection techniques in an unbiased and fully transparent way.
European Space Agency Benchmark for Anomaly Detection in Satellite Telemetry
Jun 25, 2024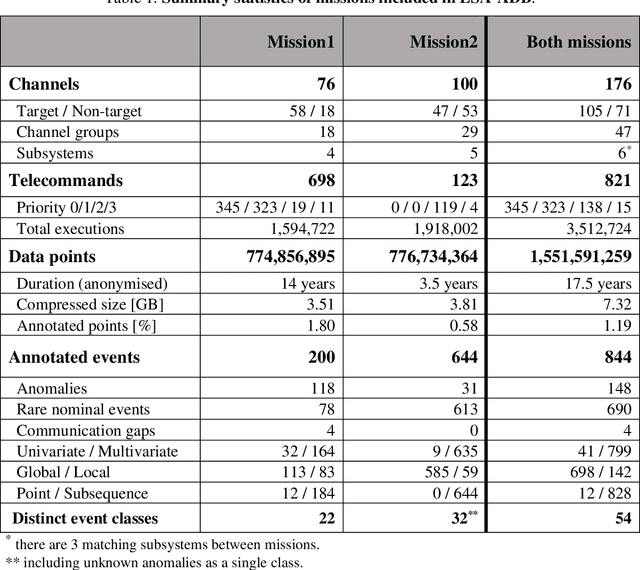



Abstract:Machine learning has vast potential to improve anomaly detection in satellite telemetry which is a crucial task for spacecraft operations. This potential is currently hampered by a lack of comprehensible benchmarks for multivariate time series anomaly detection, especially for the challenging case of satellite telemetry. The European Space Agency Benchmark for Anomaly Detection in Satellite Telemetry (ESA-ADB) aims to address this challenge and establish a new standard in the domain. It is a result of close cooperation between spacecraft operations engineers from the European Space Agency (ESA) and machine learning experts. The newly introduced ESA Anomalies Dataset contains annotated real-life telemetry from three different ESA missions, out of which two are included in ESA-ADB. Results of typical anomaly detection algorithms assessed in our novel hierarchical evaluation pipeline show that new approaches are necessary to address operators' needs. All elements of ESA-ADB are publicly available to ensure its full reproducibility.
Protein intrinsic disorder prediction using Attention U-Net and ProtTrans protein language model
Apr 11, 2024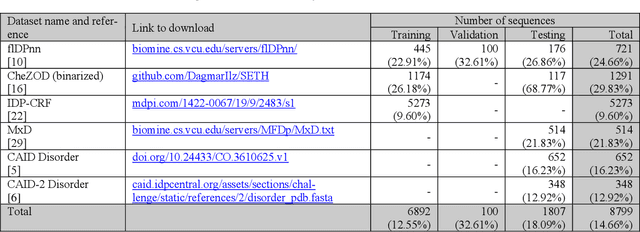
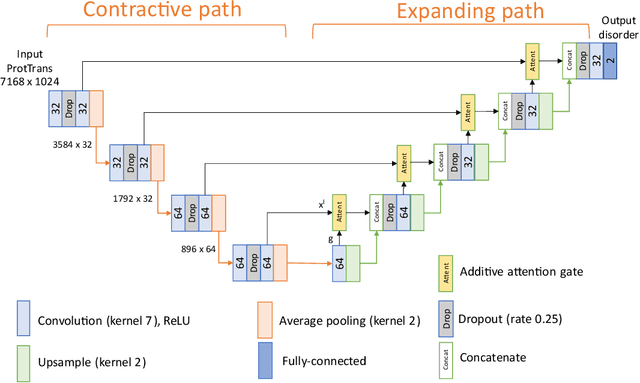
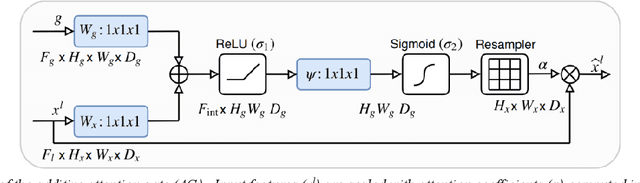
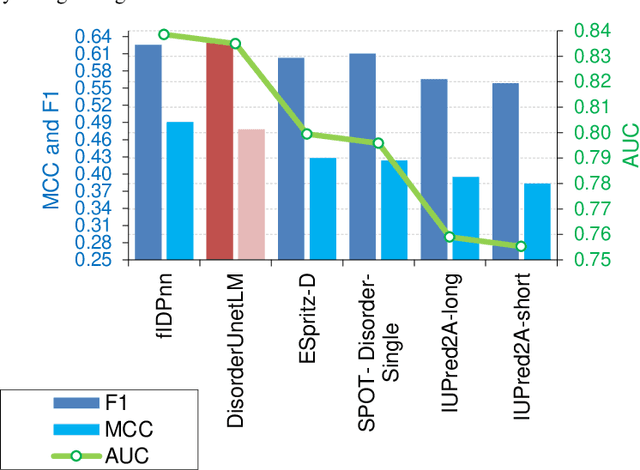
Abstract:The prediction of intrinsic disorder regions has significant implications for understanding protein function, structure, and dynamics. It can help to discover novel functions or protein-protein interactions essential to designing new drugs, therapies, or enzymes. Recently, a new generation of predictors based on protein language models is emerging. These algorithms reach state-of-the-art accuracy without calculating time-consuming multiple sequence alignments (MSAs). The article pre-sents a new protein intrinsic disorder predictor DisorderUnetLM based on the Attention U-Net convolutional neural network using features from the protein language model ProtTrans. DisorderUnetLM shows top results in the direct comparison with flDPnn and IDP-CRF predictors using MSAs and with the SETH predictor using features from the same ProtTrans model. Moreover, among 41 predictors from the latest Critical Assessment of Protein Intrinsic Disorder Prediction (CAID-2) benchmark, it ranks 9th for the Disorder-PDB subset (with ROC-AUC of 0.924) and 1st for the Disorder-NOX subset (with ROC-AUC of 0.844) which confirms its potential to perform well in the upcoming CAID-3 challenge for which Disor-derUnetLM was submitted.
Deep learning automates bidimensional and volumetric tumor burden measurement from MRI in pre- and post-operative glioblastoma patients
Sep 03, 2022



Abstract:Tumor burden assessment by magnetic resonance imaging (MRI) is central to the evaluation of treatment response for glioblastoma. This assessment is complex to perform and associated with high variability due to the high heterogeneity and complexity of the disease. In this work, we tackle this issue and propose a deep learning pipeline for the fully automated end-to-end analysis of glioblastoma patients. Our approach simultaneously identifies tumor sub-regions, including the enhancing tumor, peritumoral edema and surgical cavity in the first step, and then calculates the volumetric and bidimensional measurements that follow the current Response Assessment in Neuro-Oncology (RANO) criteria. Also, we introduce a rigorous manual annotation process which was followed to delineate the tumor sub-regions by the human experts, and to capture their segmentation confidences that are later used while training the deep learning models. The results of our extensive experimental study performed over 760 pre-operative and 504 post-operative adult patients with glioma obtained from the public database (acquired at 19 sites in years 2021-2020) and from a clinical treatment trial (47 and 69 sites for pre-/post-operative patients, 2009-2011) and backed up with thorough quantitative, qualitative and statistical analysis revealed that our pipeline performs accurate segmentation of pre- and post-operative MRIs in a fraction of the manual delineation time (up to 20 times faster than humans). The bidimensional and volumetric measurements were in strong agreement with expert radiologists, and we showed that RANO measurements are not always sufficient to quantify tumor burden.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge