Kenton M. Sanders
VTGAN: Semi-supervised Retinal Image Synthesis and Disease Prediction using Vision Transformers
Apr 14, 2021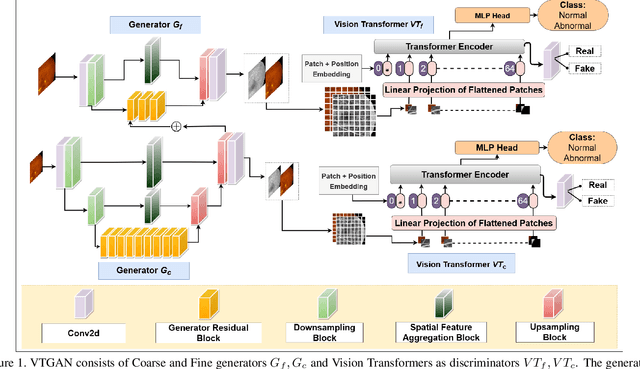
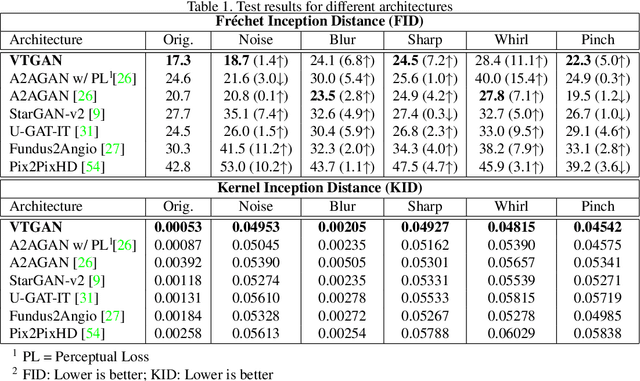
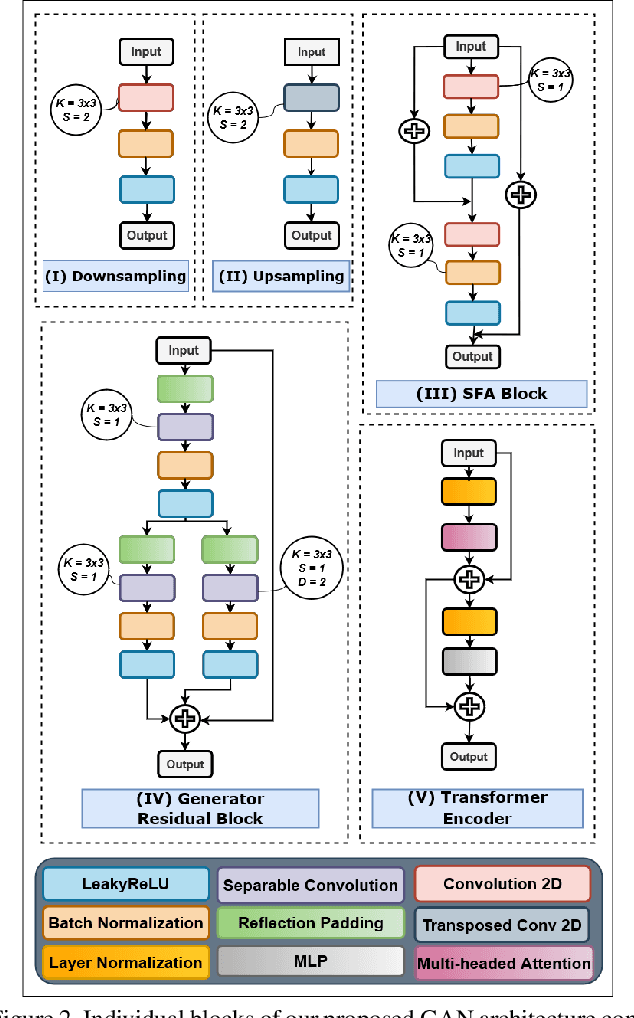
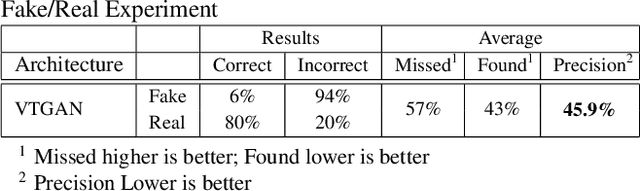
Abstract:In Fluorescein Angiography (FA), an exogenous dye is injected in the bloodstream to image the vascular structure of the retina. The injected dye can cause adverse reactions such as nausea, vomiting, anaphylactic shock, and even death. In contrast, color fundus imaging is a non-invasive technique used for photographing the retina but does not have sufficient fidelity for capturing its vascular structure. The only non-invasive method for capturing retinal vasculature is optical coherence tomography-angiography (OCTA). However, OCTA equipment is quite expensive, and stable imaging is limited to small areas on the retina. In this paper, we propose a novel conditional generative adversarial network (GAN) capable of simultaneously synthesizing FA images from fundus photographs while predicting retinal degeneration. The proposed system has the benefit of addressing the problem of imaging retinal vasculature in a non-invasive manner as well as predicting the existence of retinal abnormalities. We use a semi-supervised approach to train our GAN using multiple weighted losses on different modalities of data. Our experiments validate that the proposed architecture exceeds recent state-of-the-art generative networks for fundus-to-angiography synthesis. Moreover, our vision transformer-based discriminators generalize quite well on out-of-distribution data sets for retinal disease prediction.
RV-GAN : Retinal Vessel Segmentation from Fundus Images using Multi-scale Generative Adversarial Networks
Jan 03, 2021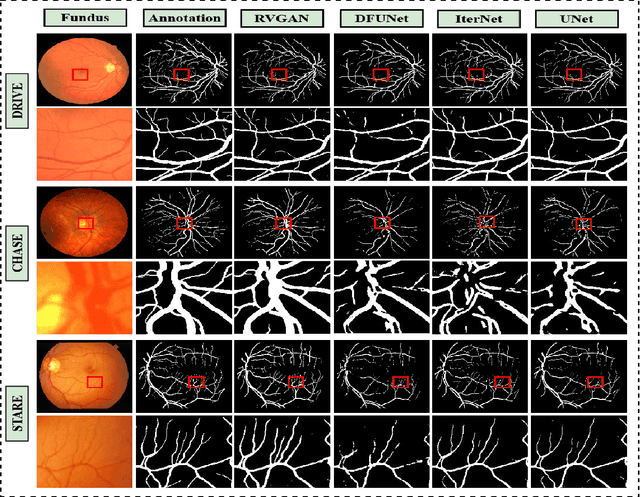
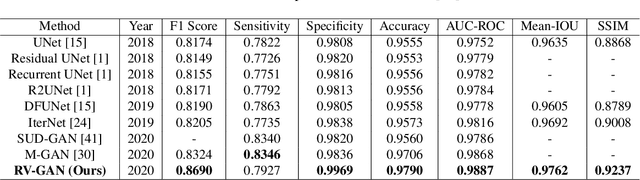
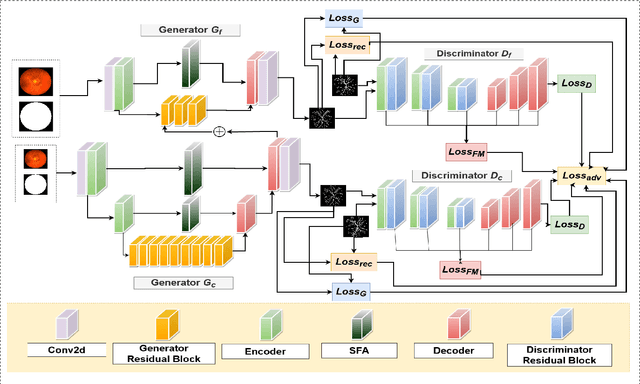
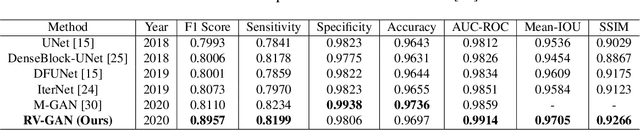
Abstract:Retinal vessel segmentation contributes significantly to the domain of retinal image analysis for the diagnosis of vision-threatening diseases. With existing techniques the generated segmentation result deteriorates when thresholded with higher confidence value. To alleviate from this, we propose RVGAN, a new multi-scale generative architecture for accurate retinal vessel segmentation. Our architecture uses two generators and two multi-scale autoencoder based discriminators, for better microvessel localization and segmentation. By combining reconstruction and weighted feature matching loss, our adversarial training scheme generates highly accurate pixel-wise segmentation of retinal vessels with threshold >= 0.5. The architecture achieves AUC of 0.9887, 0.9814, and 0.9887 on three publicly available datasets, namely DRIVE, CHASE-DB1, and STARE, respectively. Additionally, RV-GAN outperforms other architectures in two additional relevant metrics, Mean-IOU and SSIM.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge