Kaveh Laksari
Reconstructing Blood Flow in Data-Poor Regimes: A Vasculature Network Kernel for Gaussian Process Regression
Mar 14, 2024



Abstract:Blood flow reconstruction in the vasculature is important for many clinical applications. However, in clinical settings, the available data are often quite limited. For instance, Transcranial Doppler ultrasound (TCD) is a noninvasive clinical tool that is commonly used in the clinical settings to measure blood velocity waveform at several locations on brain's vasculature. This amount of data is grossly insufficient for training machine learning surrogate models, such as deep neural networks or Gaussian process regression. In this work, we propose a Gaussian process regression approach based on physics-informed kernels, enabling near-real-time reconstruction of blood flow in data-poor regimes. We introduce a novel methodology to reconstruct the kernel within the vascular network, which is a non-Euclidean space. The proposed kernel encodes both spatiotemporal and vessel-to-vessel correlations, thus enabling blood flow reconstruction in vessels that lack direct measurements. We demonstrate that any prediction made with the proposed kernel satisfies the conservation of mass principle. The kernel is constructed by running stochastic one-dimensional blood flow simulations, where the stochasticity captures the epistemic uncertainties, such as lack of knowledge about boundary conditions and uncertainties in vasculature geometries. We demonstrate the performance of the model on three test cases, namely, a simple Y-shaped bifurcation, abdominal aorta, and the Circle of Willis in the brain.
Physics-informed UNets for Discovering Hidden Elasticity in Heterogeneous Materials
Jun 07, 2023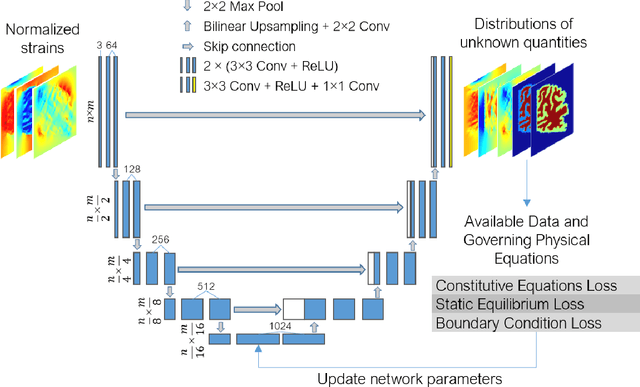
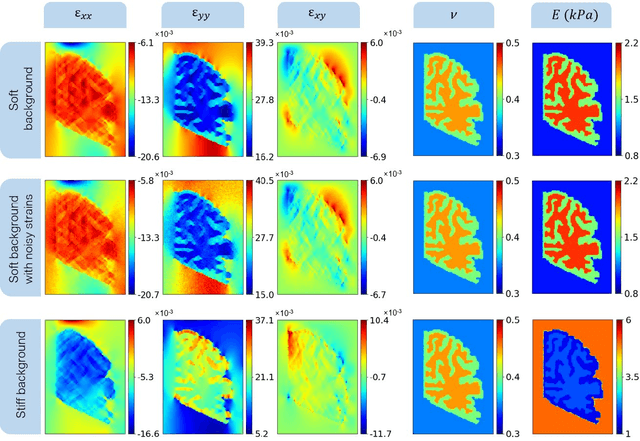
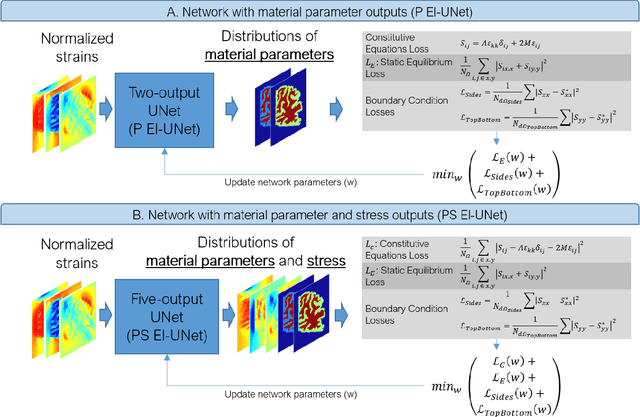
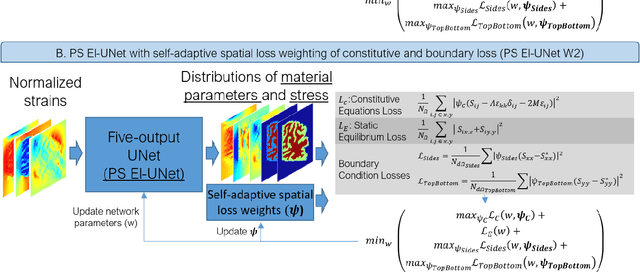
Abstract:Soft biological tissues often have complex mechanical properties due to variation in structural components. In this paper, we develop a novel UNet-based neural network model for inversion in elasticity (El-UNet) to infer the spatial distributions of mechanical parameters from strain maps as input images, normal stress boundary conditions, and domain physics information. We show superior performance, both in terms of accuracy and computational cost, by El-UNet compared to fully-connected physics-informed neural networks in estimating unknown parameters and stress distributions for isotropic linear elasticity. We characterize different variations of El-UNet and propose a self-adaptive spatial loss weighting approach. To validate our inversion models, we performed various finite-element simulations of isotropic domains with heterogenous distributions of material parameters to generate synthetic data. El-UNet is faster and more accurate than the fully-connected physics-informed implementation in resolving the distribution of unknown fields. Among the tested models, the self-adaptive spatially weighted models had the most accurate reconstructions in equal computation times. The learned spatial weighting distribution visibly corresponded to regions that the unweighted models were resolving inaccurately. Our work demonstrates a computationally efficient inversion algorithm for elasticity imaging using convolutional neural networks and presents a potential fast framework for three-dimensional inverse elasticity problems that have proven unachievable through previously proposed methods.
Cerebrovascular morphology in aging and disease -- imaging biomarkers for ischemic stroke and Alzheimers disease
Feb 14, 2022



Abstract:Background and Purpose: Altered brain vasculature is a key phenomenon in several neurologic disorders. This paper presents a quantitative assessment of vascular morphology in healthy and diseased adults including changes during aging and the anatomical variations in the Circle of Willis (CoW). Methods: We used our automatic method to segment and extract novel geometric features of the cerebral vasculature from MRA scans of 175 healthy subjects, 45 AIS, and 50 AD patients after spatial registration. This is followed by quantification and statistical analysis of vascular alterations in acute ischemic stroke (AIS) and Alzheimer's disease (AD), the biggest cerebrovascular and neurodegenerative disorders. Results: We determined that the CoW is fully formed in only 35 percent of healthy adults and found significantly increased tortuosity and fractality, with increasing age and with disease -- both AIS and AD. We also found significantly decreased vessel length, volume and number of branches in AIS patients. Lastly, we found that AD cerebral vessels exhibited significantly smaller diameter and more complex branching patterns, compared to age-matched healthy adults. These changes were significantly heightened with progression of AD from early onset to moderate-severe dementia. Conclusion: Altered vessel geometry in AIS patients shows that there is pathological morphology coupled with stroke. In AD due to pathological alterations in the endothelium or amyloid depositions leading to neuronal damage and hypoperfusion, vessel geometry is significantly altered even in mild or early dementia. The specific geometric features and quantitative comparisons demonstrate potential for using vascular morphology as a non-invasive imaging biomarker for neurologic disorders.
Physics-informed neural networks for improving cerebral hemodynamics predictions
Aug 25, 2021



Abstract:Determining brain hemodynamics plays a critical role in the diagnosis and treatment of various cerebrovascular diseases. In this work, we put forth a physics-informed deep learning framework that augments sparse clinical measurements with fast computational fluid dynamics (CFD) simulations to generate physically consistent and high spatiotemporal resolution of brain hemodynamic parameters. Transcranial Doppler (TCD) ultrasound is one of the most common techniques in the current clinical workflow that enables noninvasive and instantaneous evaluation of blood flow velocity within the cerebral arteries. However, it is spatially limited to only a handful of locations across the cerebrovasculature due to the constrained accessibility through the skull's acoustic windows. Our deep learning framework employs in-vivo real-time TCD velocity measurements at several locations in the brain and the baseline vessel cross-sectional areas acquired from 3D angiography images, and provides high-resolution maps of velocity, area, and pressure in the entire vasculature. We validated the predictions of our model against in-vivo velocity measurements obtained via 4D flow MRI scans. We then showcased the clinical significance of this technique in diagnosing the cerebral vasospasm (CVS) by successfully predicting the changes in vasospastic local vessel diameters based on corresponding sparse velocities measurements. The key finding here is that the combined effects of uncertainties in outlet boundary condition subscription and modeling physics deficiencies render the conventional purely physics-based computational models unsuccessful in recovering accurate brain hemodynamics. Nonetheless, fusing these models with clinical measurements through a data-driven approach ameliorates predictions of brain hemodynamic variables.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge