Karl-Johan Leuchowius
Bridging Generalization Gaps in High Content Imaging Through Online Self-Supervised Domain Adaptation
Nov 21, 2023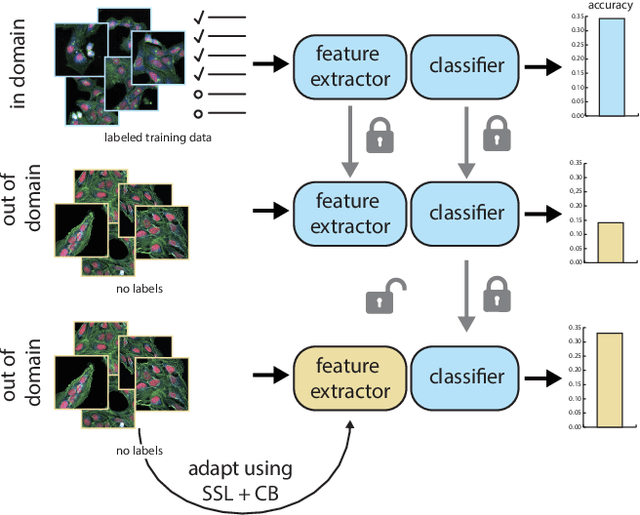
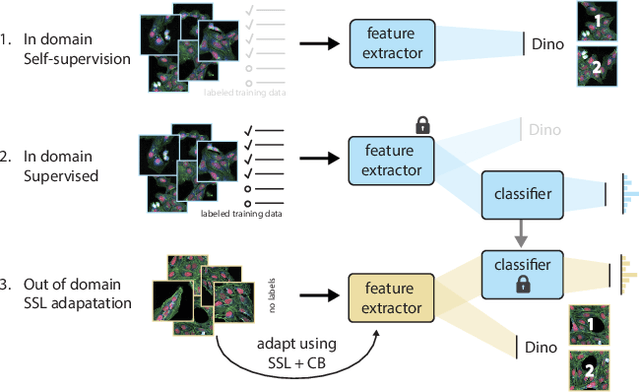
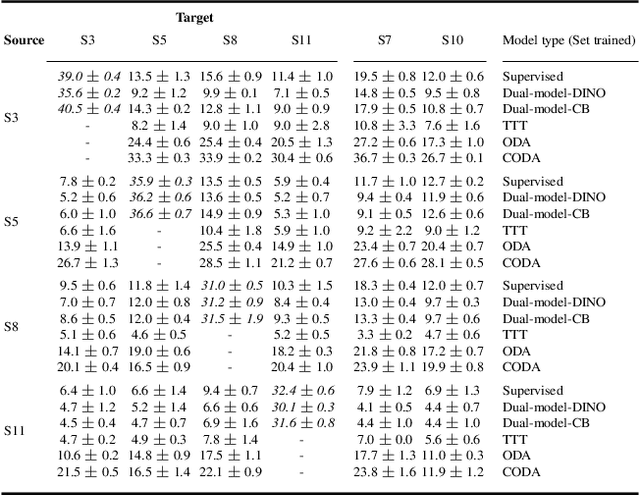
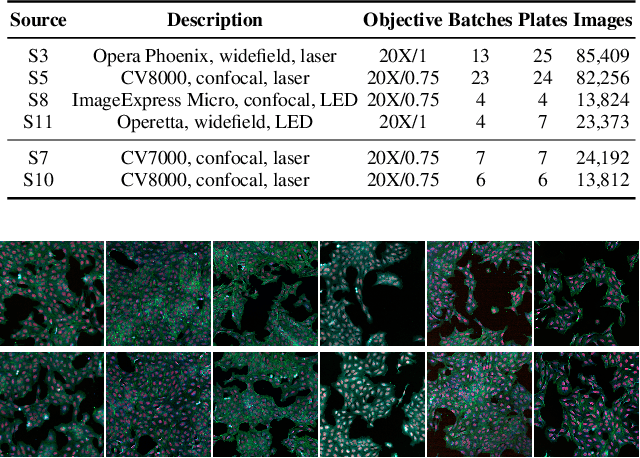
Abstract:High Content Imaging (HCI) plays a vital role in modern drug discovery and development pipelines, facilitating various stages from hit identification to candidate drug characterization. Applying machine learning models to these datasets can prove challenging as they typically consist of multiple batches, affected by experimental variation, especially if different imaging equipment have been used. Moreover, as new data arrive, it is preferable that they are analyzed in an online fashion. To overcome this, we propose CODA, an online self-supervised domain adaptation approach. CODA divides the classifier's role into a generic feature extractor and a task-specific model. We adapt the feature extractor's weights to the new domain using cross-batch self-supervision while keeping the task-specific model unchanged. Our results demonstrate that this strategy significantly reduces the generalization gap, achieving up to a 300% improvement when applied to data from different labs utilizing different microscopes. CODA can be applied to new, unlabeled out-of-domain data sources of different sizes, from a single plate to multiple experimental batches.
Metadata-guided Consistency Learning for High Content Images
Dec 22, 2022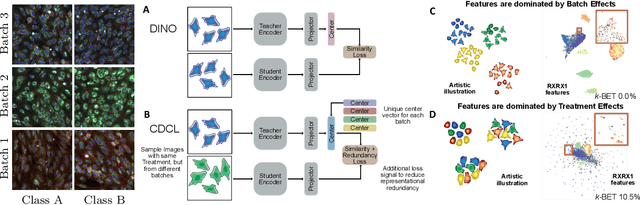
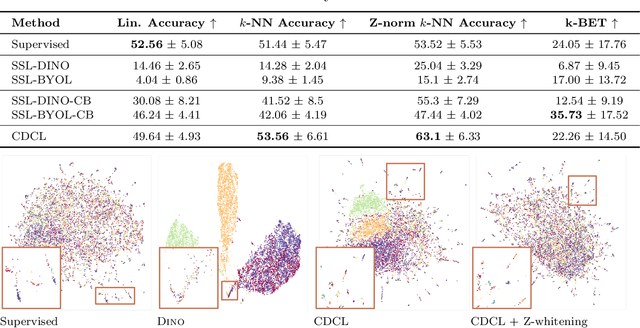

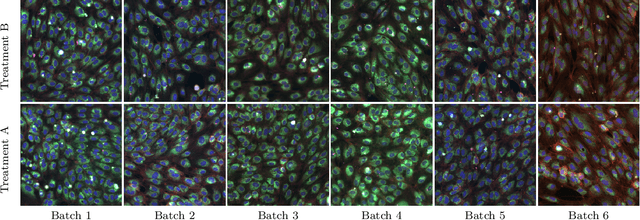
Abstract:High content imaging assays can capture rich phenotypic response data for large sets of compound treatments, aiding in the characterization and discovery of novel drugs. However, extracting representative features from high content images that can capture subtle nuances in phenotypes remains challenging. The lack of high-quality labels makes it difficult to achieve satisfactory results with supervised deep learning. Self-Supervised learning methods, which learn from automatically generated labels has shown great success on natural images, offer an attractive alternative also to microscopy images. However, we find that self-supervised learning techniques underperform on high content imaging assays. One challenge is the undesirable domain shifts present in the data known as batch effects, which may be caused by biological noise or uncontrolled experimental conditions. To this end, we introduce Cross-Domain Consistency Learning (CDCL), a novel approach that is able to learn in the presence of batch effects. CDCL enforces the learning of biological similarities while disregarding undesirable batch-specific signals, which leads to more useful and versatile representations. These features are organised according to their morphological changes and are more useful for downstream tasks - such as distinguishing treatments and mode of action.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge