Julian Arz
3D Cell Nuclei Segmentation with Balanced Graph Partitioning
Feb 17, 2017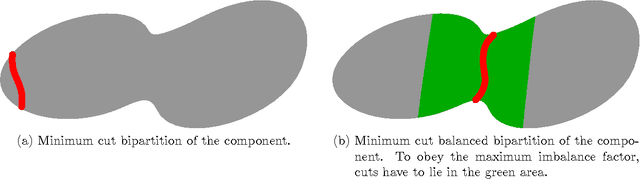
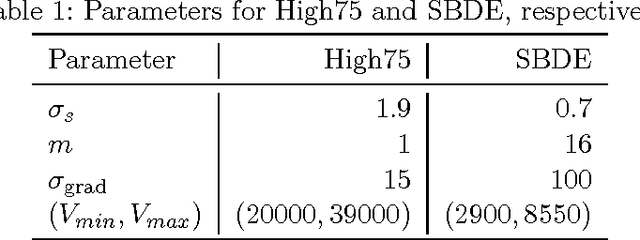
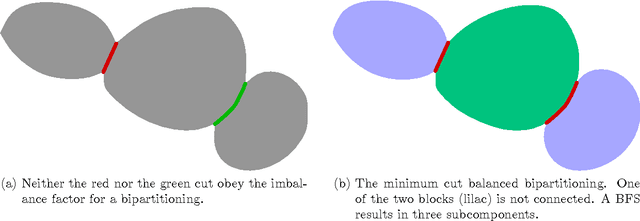

Abstract:Cell nuclei segmentation is one of the most important tasks in the analysis of biomedical images. With ever-growing sizes and amounts of three-dimensional images to be processed, there is a need for better and faster segmentation methods. Graph-based image segmentation has seen a rise in popularity in recent years, but is seen as very costly with regard to computational demand. We propose a new segmentation algorithm which overcomes these limitations. Our method uses recursive balanced graph partitioning to segment foreground components of a fast and efficient binarization. We construct a model for the cell nuclei to guide the partitioning process. Our algorithm is compared to other state-of-the-art segmentation algorithms in an experimental evaluation on two sets of realistically simulated inputs. Our method is faster, has similar or better quality and an acceptable memory overhead.
Generating Semi-Synthetic Validation Benchmarks for Embryomics
Apr 17, 2016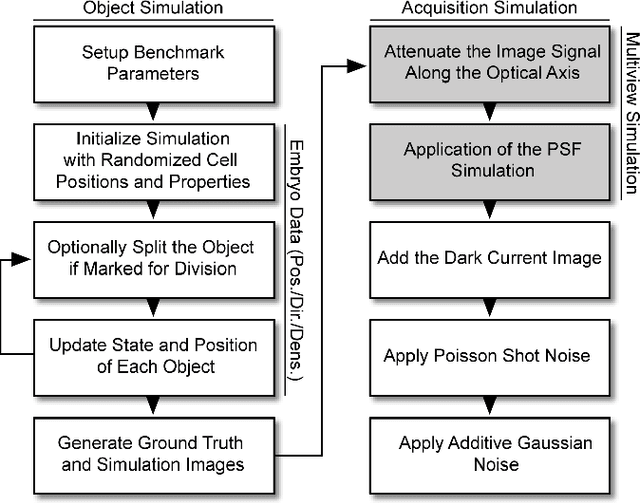
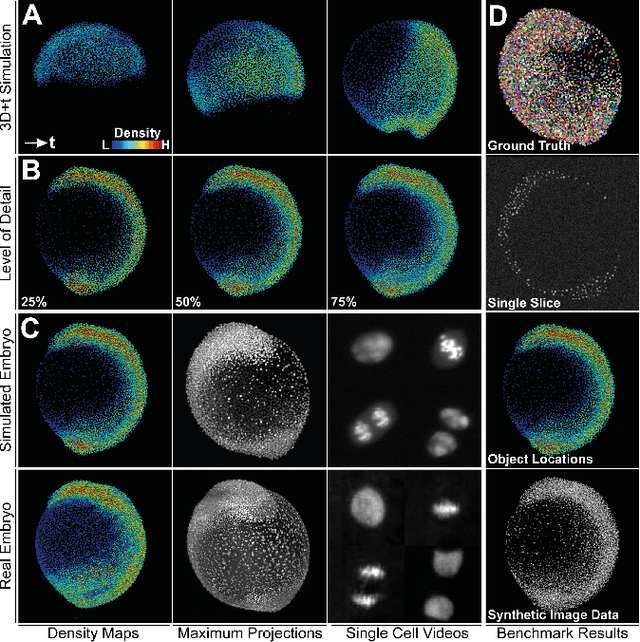
Abstract:Systematic validation is an essential part of algorithm development. The enormous dataset sizes and the complexity observed in many recent time-resolved 3D fluorescence microscopy imaging experiments, however, prohibit a comprehensive manual ground truth generation. Moreover, existing simulated benchmarks in this field are often too simple or too specialized to sufficiently validate the observed image analysis problems. We present a new semi-synthetic approach to generate realistic 3D+t benchmarks that combines challenging cellular movement dynamics of real embryos with simulated fluorescent nuclei and artificial image distortions including various parametrizable options like cell numbers, acquisition deficiencies or multiview simulations. We successfully applied the approach to simulate the development of a zebrafish embryo with thousands of cells over 14 hours of its early existence.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge