João Lourenço Silva
Using Soft Labels to Model Uncertainty in Medical Image Segmentation
Sep 26, 2021
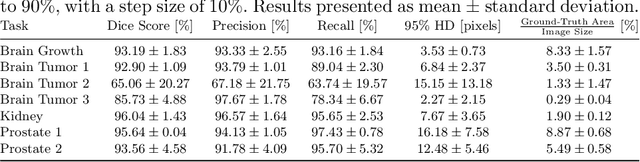

Abstract:Medical image segmentation is inherently uncertain. For a given image, there may be multiple plausible segmentation hypotheses, and physicians will often disagree on lesion and organ boundaries. To be suited to real-world application, automatic segmentation systems must be able to capture this uncertainty and variability. Thus far, this has been addressed by building deep learning models that, through dropout, multiple heads, or variational inference, can produce a set - infinite, in some cases - of plausible segmentation hypotheses for any given image. However, in clinical practice, it may not be practical to browse all hypotheses. Furthermore, recent work shows that segmentation variability plateaus after a certain number of independent annotations, suggesting that a large enough group of physicians may be able to represent the whole space of possible segmentations. Inspired by this, we propose a simple method to obtain soft labels from the annotations of multiple physicians and train models that, for each image, produce a single well-calibrated output that can be thresholded at multiple confidence levels, according to each application's precision-recall requirements. We evaluated our method on the MICCAI 2021 QUBIQ challenge, showing that it performs well across multiple medical image segmentation tasks, produces well-calibrated predictions, and, on average, performs better at matching physicians' predictions than other physicians.
Encoder-Decoder Architectures for Clinically Relevant Coronary Artery Segmentation
Jun 21, 2021



Abstract:Coronary X-ray angiography is a crucial clinical procedure for the diagnosis and treatment of coronary artery disease, which accounts for roughly 16% of global deaths every year. However, the images acquired in these procedures have low resolution and poor contrast, making lesion detection and assessment challenging. Accurate coronary artery segmentation not only helps mitigate these problems, but also allows the extraction of relevant anatomical features for further analysis by quantitative methods. Although automated segmentation of coronary arteries has been proposed before, previous approaches have used non-optimal segmentation criteria, leading to less useful results. Most methods either segment only the major vessel, discarding important information from the remaining ones, or segment the whole coronary tree based mostly on contrast information, producing a noisy output that includes vessels that are not relevant for diagnosis. We adopt a better-suited clinical criterion and segment vessels according to their clinical relevance. Additionally, we simultaneously perform catheter segmentation, which may be useful for diagnosis due to the scale factor provided by the catheter's known diameter, and is a task that has not yet been performed with good results. To derive the optimal approach, we conducted an extensive comparative study of encoder-decoder architectures trained on a combination of focal loss and a variant of generalized dice loss. Based on the EfficientNet and the UNet++ architectures, we propose a line of efficient and high-performance segmentation models using a new decoder architecture, the EfficientUNet++, whose best-performing version achieved average dice scores of 0.8904 and 0.7526 for the artery and catheter classes, respectively, and an average generalized dice score of 0.9234.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge