Jiri Damborsky
Revealing data leakage in protein interaction benchmarks
Apr 16, 2024Abstract:In recent years, there has been remarkable progress in machine learning for protein-protein interactions. However, prior work has predominantly focused on improving learning algorithms, with less attention paid to evaluation strategies and data preparation. Here, we demonstrate that further development of machine learning methods may be hindered by the quality of existing train-test splits. Specifically, we find that commonly used splitting strategies for protein complexes, based on protein sequence or metadata similarity, introduce major data leakage. This may result in overoptimistic evaluation of generalization, as well as unfair benchmarking of the models, biased towards assessing their overfitting capacity rather than practical utility. To overcome the data leakage, we recommend constructing data splits based on 3D structural similarity of protein-protein interfaces and suggest corresponding algorithms. We believe that addressing the data leakage problem is critical for further progress in this research area.
Learning to design protein-protein interactions with enhanced generalization
Oct 27, 2023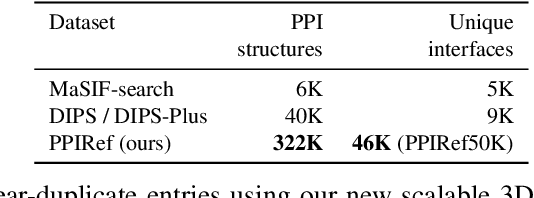
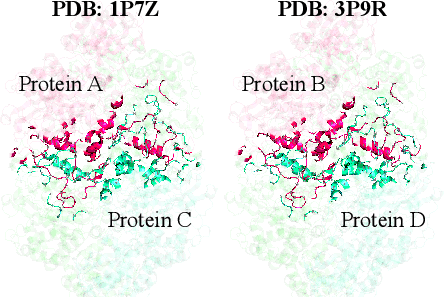
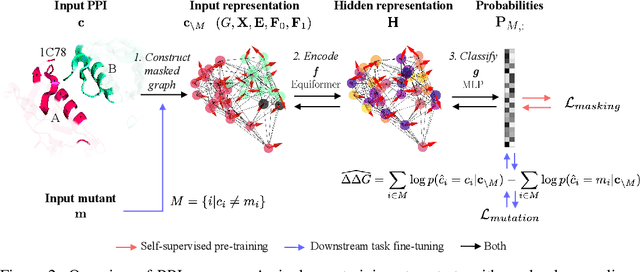
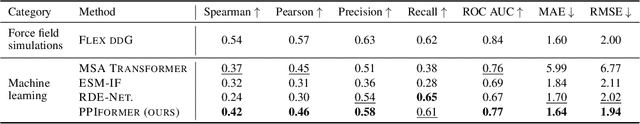
Abstract:Discovering mutations enhancing protein-protein interactions (PPIs) is critical for advancing biomedical research and developing improved therapeutics. While machine learning approaches have substantially advanced the field, they often struggle to generalize beyond training data in practical scenarios. The contributions of this work are three-fold. First, we construct PPIRef, the largest and non-redundant dataset of 3D protein-protein interactions, enabling effective large-scale learning. Second, we leverage the PPIRef dataset to pre-train PPIformer, a new SE(3)-equivariant model generalizing across diverse protein-binder variants. We fine-tune PPIformer to predict effects of mutations on protein-protein interactions via a thermodynamically motivated adjustment of the pre-training loss function. Finally, we demonstrate the enhanced generalization of our new PPIformer approach by outperforming other state-of-the-art methods on new, non-leaking splits of standard labeled PPI mutational data and independent case studies optimizing a human antibody against SARS-CoV-2 and increasing the thrombolytic activity of staphylokinase.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge