Jane Crowley
Ultra-miniature dual-wavelength spatial frequency domain imaging for micro-endoscopy
Jun 06, 2023Abstract:There is a need for a cost-effective, quantitative imaging tool that can be deployed endoscopically to better detect early stage gastrointestinal cancers. Spatial frequency domain imaging (SFDI) is a low-cost imaging technique that produces near-real time, quantitative maps of absorption and reduced scattering coefficients, but most implementations are bulky and suitable only for use outside the body. We present an ultra-miniature SFDI system comprised of an optical fiber array (diameter 0.125 mm) and a micro camera (1 x 1 mm package) displacing conventionally bulky components, in particular the projector. The prototype has outer diameter 3 mm, but the individual components dimensions could permit future packaging to < 1.5 mm diameter. We develop a phase-tracking algorithm to rapidly extract images with fringe projections at 3 equispaced phase shifts in order to perform SFDI demodulation. To validate performance, we first demonstrate comparable recovery of quantitative optical properties between our ultra-miniature system and a conventional bench-top SFDI system with agreement of 15% and 6% for absorption and reduced scattering respectively. Next, we demonstrate imaging of absorption and reduced scattering of tissue-mimicking phantoms providing enhanced contrast between simulated tissue types (healthy and tumour), done simultaneously at wavelengths of 515 nm and 660 nm. This device shows promise as a cost-effective, quantitative imaging tool to detect variations in optical absorption and scattering as indicators of cancer.
Designing and simulating realistic spatial frequency domain imaging systems using open-source 3D rendering software
Feb 24, 2023



Abstract:Spatial frequency domain imaging (SFDI) is a low-cost imaging technique that can deliver real-time maps of absorption and reduced scattering coefficients. However, there are a wide range of imaging geometries that practical SFDI systems must cope with including imaging flat samples ex vivo, imaging inside tubular lumen in vivo such as in an endoscopy, and measuring tumours or polyps of varying shapes, sizes and optical properties. There is a need for a design and simulation tool to accelerate design and fabrication of new SFDI systems. We present such a system implemented using open-source 3D design and ray-tracing software Blender that is capable of simulating media with realistic optical properties (mimicking healthy and cancerous tissue), a wide variety of shapes and size, and in both planar and tubular imaging geometries. We first demonstrate quantitative agreement between Monte-Carlo simulated scattering and absorption coefficients and those measured from our Blender system. Next, we show the ability of the system to simulate absorption, scattering and shape for flat samples with small simulated tumours and show that the improved contrast associated with SFDI is reproduced. Finally, to demonstrate the versatility of the system as a design tool we show that it can be used to generate a custom look-up-table for mapping from modulation amplitude values to absorption and scattering values in a tubular geometry, simulating a lumen. As a demonstrative example we show that longitudinal sectioning of the tube, with separate look-up tables for each section, significantly improves accuracy of SFDI, representing an important design insight for future systems. We therefore anticipate our simulation system will significantly aid in the design and development of novel SFDI systems, especially as such systems are miniaturised for deployment in endoscopic and laparoscopic systems.
Training Generative Adversarial Networks for Optical Property Mapping using Synthetic Image Data
Mar 15, 2022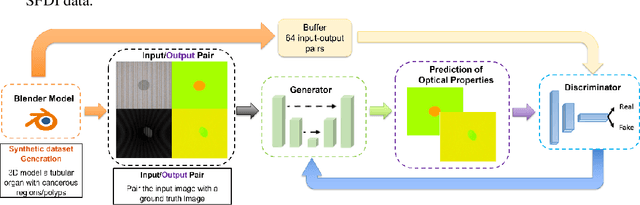
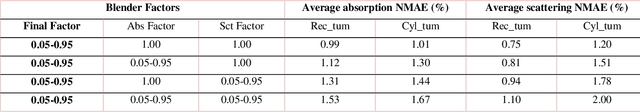
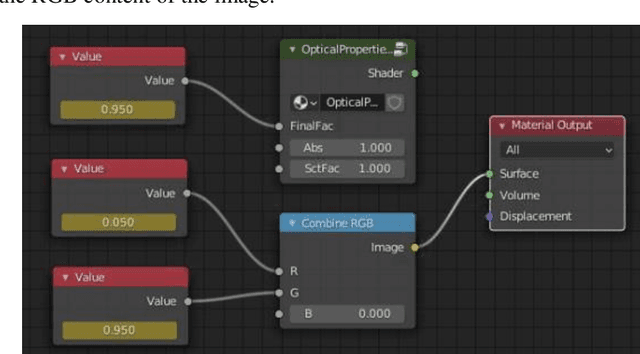
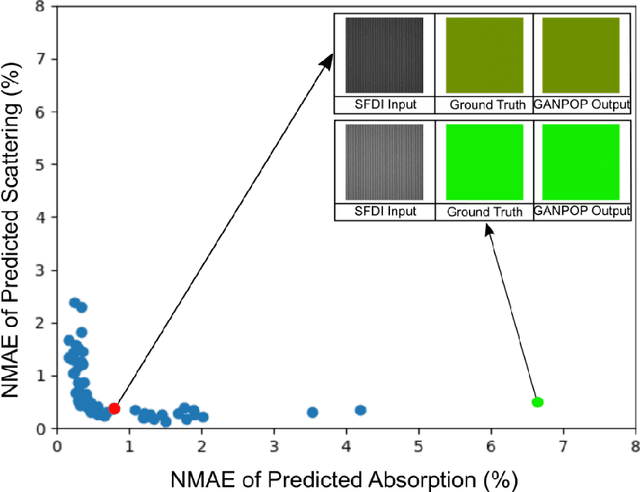
Abstract:We demonstrate training of a Generative Adversarial Network (GAN) for prediction of optical property maps (scattering and absorption) using spatial frequency domain imaging (SFDI) image data sets generated synthetically with free open-source 3D modelling and rendering software, Blender. The flexibility of Blender is exploited to simulate 3 models with real-life relevance to clinical SFDI of diseased tissue: flat samples, flat samples with spheroidal tumours and cylindrical samples with spheroidal tumours representing imaging inside a tubular organ e.g. the gastro-intestinal tract. In all 3 scenarios we show the GAN provides accurate reconstruction of optical properties from single SFDI images with mean normalised error ranging from 1-1.2% for absorption and 0.7-1.2% for scattering, resulting in visually improved contrast for tumour spheroid structures. This compares favourably with 25% absorption error and 10% scattering error achieved using GANs on experimental SFDI data. However, some of this improvement is due to lower noise and availability of perfect ground truths so we therefore cross-validate our synthetically-trained GAN with a GAN trained on experimental data and observe visually accurate results with error of <40% for absorption and <25% for scattering, due largely to the presence of spatial frequency mismatch artefacts. Our synthetically trained GAN is therefore highly relevant to real experimental samples, but provides significant added benefits of large training datasets, perfect ground-truths and the ability to test realistic imaging geometries, e.g. inside cylinders, for which no conventional single-shot demodulation algorithms exist. In future we expect that application of techniques such as domain adaptation or training on hybrid real-synthetic datasets will create a powerful tool for fast, accurate production of optical property maps from real clinical imaging systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge