Jacob T. Robinson
Magnetoelectric Bio-Implants Powered and Programmed by a Single Transmitter for Coordinated Multisite Stimulation
Dec 31, 2021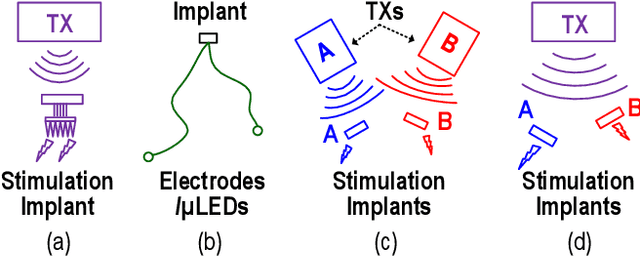
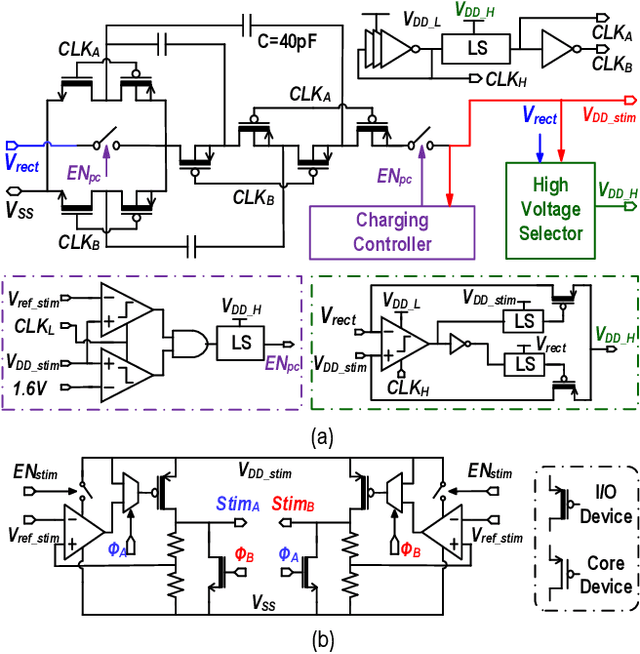
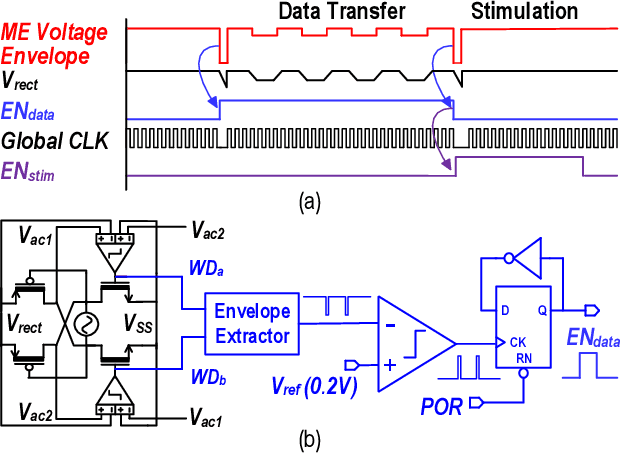
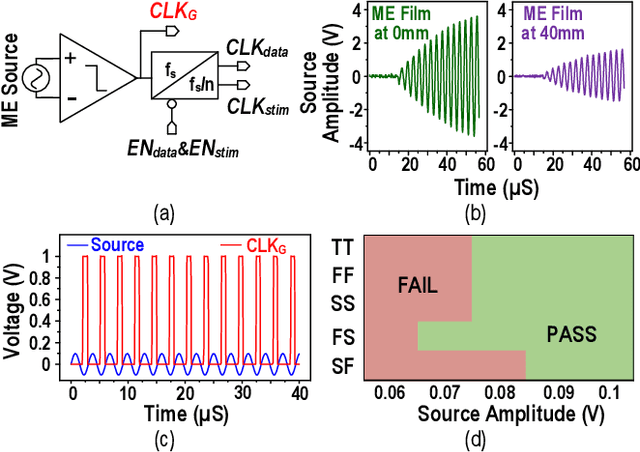
Abstract:This article presents a hardware platform including stimulating implants wirelessly powered and controlled by a shared transmitter (TX) for coordinated leadless multisite stimulation. The adopted novel single-TX, multiple-implant structure can flexibly deploy stimuli, improve system efficiency, easily scale stimulating channel quantity, and relieve efforts in device synchronization. In the proposed system, a wireless link leveraging magnetoelectric (ME) effect is co-designed with a robust and efficient system-on-chip (SoC) to enable reliable operation and individual programming of every implant. Each implant integrates a 0.8-mm2 chip, a 6-mm2 ME film, and an energy storage capacitor within a 6.2-mm3 size. ME power transfer is capable of safely transmitting milliwatt power to devices placed several centimeters away from the TX coil, maintaining good efficiency with size constraints, and tolerating 60 degree, 1.5-cm misalignment in angular and lateral movement. The SoC robustly operates with 2-V source amplitude variations that spans a 40-mm TX-implant distance change, realizes individual addressability through physical unclonable function (PUF) IDs, and achieves 90% efficiency for 1.5-3.5-V stimulation with fully programmable stimulation parameters.
* This paper has been published in IEEE Journal of Solid-State Circuits, 2021
MagNI: A Magnetoelectrically Powered and Controlled Wireless Neurostimulating Implant
Jul 07, 2021



Abstract:This paper presents the first wireless and programmable neural stimulator leveraging magnetoelectric (ME) effects for power and data transfer. Thanks to low tissue absorption, low misalignment sensitivity and high power transfer efficiency, the ME effect enables safe delivery of high power levels (a few milliwatts) at low resonant frequencies (~250 kHz) to mm-sized implants deep inside the body (30-mm depth). The presented MagNI (Magnetoelectric Neural Implant) consists of a 1.5-mm$^2$ 180-nm CMOS chip, an in-house built 4x2 mm ME film, an energy storage capacitor, and on-board electrodes on a flexible polyimide substrate with a total volume of 8.2 mm$^3$ . The chip with a power consumption of 23.7 $\mu$W includes robust system control and data recovery mechanisms under source amplitude variations (1-V variation tolerance). The system delivers fully-programmable bi-phasic current-controlled stimulation with patterns covering 0.05-to-1.5-mA amplitude, 64-to-512-$\mu$s pulse width, and 0-to-200Hz repetition frequency for neurostimulation.
* This work has been accepted to 2020 IEEE Transactions on Biomedical Circuits and Systems (TBioCAS)
High Resolution, Deep Imaging Using Confocal Time-of-flight Diffuse Optical Tomography
Jan 27, 2021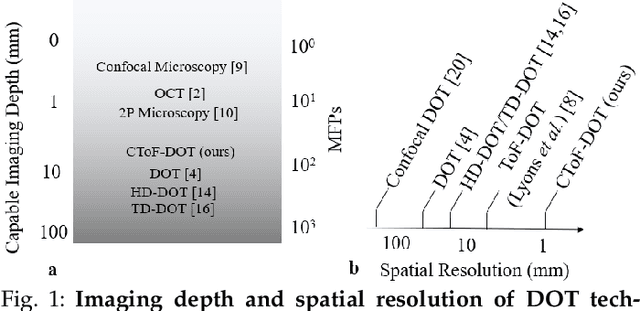
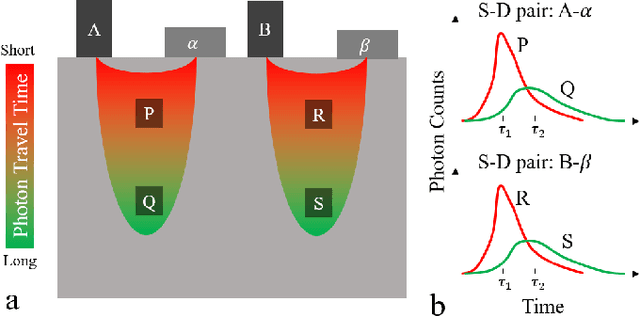

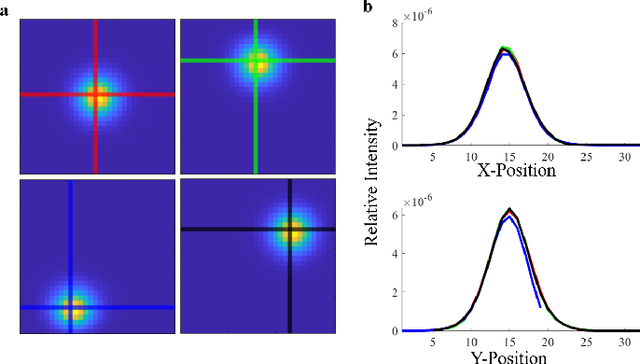
Abstract:Light scattering by tissue severely limits both how deep beneath the surface one can image, and at what spatial resolution one can obtain from these images. Diffuse optical tomography (DOT) has emerged as one of the most powerful techniques for imaging deep within tissue -- well beyond the conventional $\sim$ 10-15 mean scattering lengths tolerated by ballistic imaging techniques such as confocal and two-photon microscopy. Unfortunately, existing DOT systems are quite limited and achieve only centimeter-scale resolution. Furthermore, they also suffer from slow acquisition times and extremely slow reconstruction speeds making real-time imaging infeasible. We show that time-of-flight diffuse optical tomography (ToF-DOT) and its confocal variant (CToF-DOT), by exploiting the photon travel time information, allow us to achieve millimeter spatial resolution in the highly scattered diffusion regime ($>50$ mean free paths). In addition, we demonstrate that two additional innovations: focusing on confocal measurements, and multiplexing the illumination sources allow us to significantly reduce the scan time to acquire measurements. Finally, we also rely on a novel convolutional approximation that allows us to develop a fast reconstruction algorithm achieving a 100 $\times$ speedup in reconstruction time compared to traditional DOT reconstruction techniques. Together, we believe that these technical advances, serve as the first step towards real-time, millimeter resolution, deep tissue imaging using diffuse optical tomography.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge