Jacob Granley
Human-in-the-Loop Optimization for Deep Stimulus Encoding in Visual Prostheses
Jun 16, 2023Abstract:Neuroprostheses show potential in restoring lost sensory function and enhancing human capabilities, but the sensations produced by current devices often seem unnatural or distorted. Exact placement of implants and differences in individual perception lead to significant variations in stimulus response, making personalized stimulus optimization a key challenge. Bayesian optimization could be used to optimize patient-specific stimulation parameters with limited noisy observations, but is not feasible for high-dimensional stimuli. Alternatively, deep learning models can optimize stimulus encoding strategies, but typically assume perfect knowledge of patient-specific variations. Here we propose a novel, practically feasible approach that overcomes both of these fundamental limitations. First, a deep encoder network is trained to produce optimal stimuli for any individual patient by inverting a forward model mapping electrical stimuli to visual percepts. Second, a preferential Bayesian optimization strategy utilizes this encoder to optimize patient-specific parameters for a new patient, using a minimal number of pairwise comparisons between candidate stimuli. We demonstrate the viability of this approach on a novel, state-of-the-art visual prosthesis model. We show that our approach quickly learns a personalized stimulus encoder, leads to dramatic improvements in the quality of restored vision, and is robust to noisy patient feedback and misspecifications in the underlying forward model. Overall, our results suggest that combining the strengths of deep learning and Bayesian optimization could significantly improve the perceptual experience of patients fitted with visual prostheses and may prove a viable solution for a range of neuroprosthetic technologies.
Explaining V1 Properties with a Biologically Constrained Deep Learning Architecture
May 25, 2023Abstract:Convolutional neural networks (CNNs) have recently emerged as promising models of the ventral visual stream, despite their lack of biological specificity. While current state-of-the-art models of the primary visual cortex (V1) have surfaced from training with adversarial examples and extensively augmented data, these models are still unable to explain key neural properties observed in V1 that arise from biological circuitry. To address this gap, we systematically incorporated neuroscience-derived architectural components into CNNs to identify a set of mechanisms and architectures that comprehensively explain neural activity in V1. We show drastic improvements in model-V1 alignment driven by the integration of architectural components that simulate center-surround antagonism, local receptive fields, tuned normalization, and cortical magnification. Upon enhancing task-driven CNNs with a collection of these specialized components, we uncover models with latent representations that yield state-of-the-art explanation of V1 neural activity and tuning properties. Our results highlight an important advancement in the field of NeuroAI, as we systematically establish a set of architectural components that contribute to unprecedented explanation of V1. The neuroscience insights that could be gleaned from increasingly accurate in-silico models of the brain have the potential to greatly advance the fields of both neuroscience and artificial intelligence.
Adapting Brain-Like Neural Networks for Modeling Cortical Visual Prostheses
Sep 27, 2022


Abstract:Cortical prostheses are devices implanted in the visual cortex that attempt to restore lost vision by electrically stimulating neurons. Currently, the vision provided by these devices is limited, and accurately predicting the visual percepts resulting from stimulation is an open challenge. We propose to address this challenge by utilizing 'brain-like' convolutional neural networks (CNNs), which have emerged as promising models of the visual system. To investigate the feasibility of adapting brain-like CNNs for modeling visual prostheses, we developed a proof-of-concept model to predict the perceptions resulting from electrical stimulation. We show that a neurologically-inspired decoding of CNN activations produces qualitatively accurate phosphenes, comparable to phosphenes reported by real patients. Overall, this is an essential first step towards building brain-like models of electrical stimulation, which may not just improve the quality of vision provided by cortical prostheses but could also further our understanding of the neural code of vision.
A Hybrid Neural Autoencoder for Sensory Neuroprostheses and Its Applications in Bionic Vision
May 26, 2022
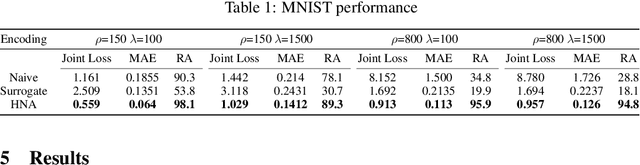


Abstract:Sensory neuroprostheses are emerging as a promising technology to restore lost sensory function or augment human capacities. However, sensations elicited by current devices often appear artificial and distorted. Although current models can often predict the neural or perceptual response to an electrical stimulus, an optimal stimulation strategy solves the inverse problem: what is the required stimulus to produce a desired response? Here we frame this as an end-to-end optimization problem, where a deep neural network encoder is trained to invert a known, fixed forward model that approximates the underlying biological system. As a proof of concept, we demonstrate the effectiveness of our hybrid neural autoencoder (HNA) on the use case of visual neuroprostheses. We found that HNA is able to produce high-fidelity stimuli from the MNIST and COCO datasets that outperform conventional encoding strategies and surrogate techniques across all tested conditions. Overall this is an important step towards the long-standing challenge of restoring high-quality vision to people living with incurable blindness and may prove a promising solution for a variety of neuroprosthetic technologies.
U-Net with Hierarchical Bottleneck Attention for Landmark Detection in Fundus Images of the Degenerated Retina
Jul 09, 2021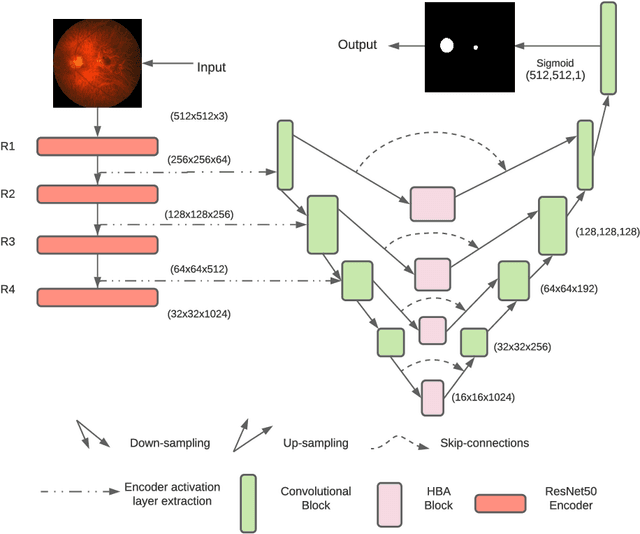
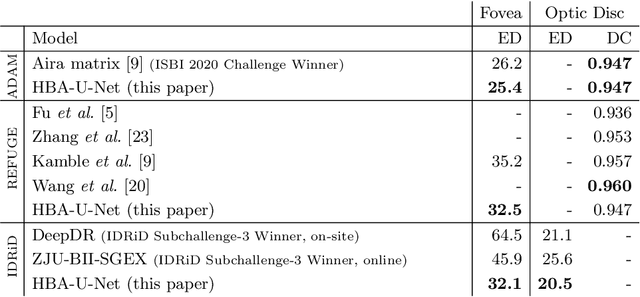
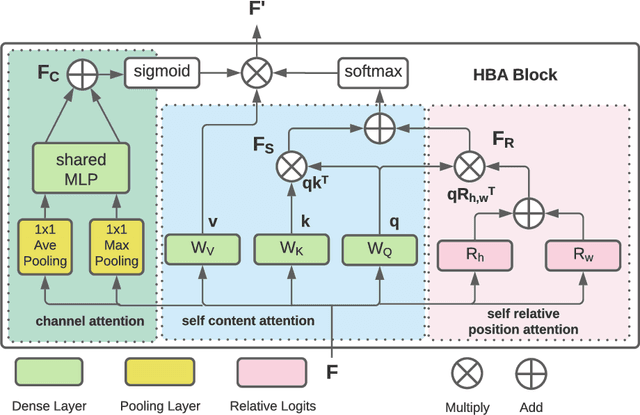

Abstract:Fundus photography has routinely been used to document the presence and severity of retinal degenerative diseases such as age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy (DR) in clinical practice, for which the fovea and optic disc (OD) are important retinal landmarks. However, the occurrence of lesions, drusen, and other retinal abnormalities during retinal degeneration severely complicates automatic landmark detection and segmentation. Here we propose HBA-U-Net: a U-Net backbone enriched with hierarchical bottleneck attention. The network consists of a novel bottleneck attention block that combines and refines self-attention, channel attention, and relative-position attention to highlight retinal abnormalities that may be important for fovea and OD segmentation in the degenerated retina. HBA-U-Net achieved state-of-the-art results on fovea detection across datasets and eye conditions (ADAM: Euclidean Distance (ED) of 25.4 pixels, REFUGE: 32.5 pixels, IDRiD: 32.1 pixels), on OD segmentation for AMD (ADAM: Dice Coefficient (DC) of 0.947), and on OD detection for DR (IDRiD: ED of 20.5 pixels). Our results suggest that HBA-U-Net may be well suited for landmark detection in the presence of a variety of retinal degenerative diseases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge