Issam El Naqa
Department of Machine Learning, Moffitt Cancer Center, Tampa, FL, USA
Evaluation of QCNN-LSTM for Disability Forecasting in Multiple Sclerosis Using Sequential Multisequence MRI
Jan 22, 2024Abstract:Introduction Quantum Convolutional Neural Network (QCNN)-Long Short-Term Memory (LSTM) models were studied to provide sequential relationships for each timepoint in MRIs of patients with Multiple Sclerosis (MS). In this pilot study, we compared three QCNN-LSTM models for binary classification of MS disability benchmarked against classical neural network architectures. Our hypothesis is that quantum models will provide competitive performance. Methods Matrix Product State (MPS), reverse Multistate Entanglement Renormalization Ansatz (MERA), and Tree-Tensor Network (TTN) circuits were paired with LSTM layer to process near-annual MRI data of patients diagnosed with MS. These were benchmarked against a Visual Geometry Group (VGG)-LSTM and a Video Vision Transformer (ViViT). Predicted logits were measured against ground truth labels of each patient's Extended Disability Severity Score (EDSS) using binary cross-entropy loss. Training/validation/holdout testing was partitioned using 5-fold cross validation with a total split of 60:20:20. Levene's test of variance was used to measure statistical difference and Student's t-test for paired model differences in mean. Results The MPS-LSTM, reverse MERA-LSTM, and TTN-LSTM had holdout testing ROC-AUC of 0.70, 0.77, and 0.81, respectively (p-value 0.915). VGG16-LSTM and ViViT performed similarly with ROC-AUC of 0.73 and 0.77, respectively (p-value 0.631). Overall variance and mean were not statistically significant (p-value 0.713), however, time to train was significantly faster for the QCNN-LSTMs (39.4 sec per fold vs. 224 and 218, respectively, p-value <0.001). Conclusion QCNN-LSTM models perform competitively to their classical counterparts with greater efficiency in train time. Clinically, these can add value in terms of efficiency to time-dependent deep learning prediction of disease progression based upon medical imaging.
Modeling non-genetic information dynamics in cells using reservoir computing
Dec 13, 2023Abstract:Virtually all cells use energy and ion-specific membrane pumps to maintain large transmembrane gradients of Na$^+$, K$^+$, Cl$^-$, Mg$^{++}$, and Ca$^{++}$. Although they consume up to 1/3 of a cell's energy budget, the corresponding evolutionary benefit of transmembrane ion gradients remain unclear. Here, we propose that ion gradients enable a dynamic and versatile biological system that acquires, analyzes, and responds to environmental information. We hypothesize environmental signals are transmitted into the cell by ion fluxes along pre-existing gradients through gated ion-specific membrane channels. The consequent changes of cytoplasmic ion concentration can generate a local response and orchestrate global or regional responses through wire-like ion fluxes along pre-existing and self-assembling cytoskeleton to engage the endoplasmic reticulum, mitochondria, and nucleus. Here, we frame our hypothesis through a quasi-physical (Cell-Reservoir) model that treats intra-cellular ion-based information dynamics as a sub-cellular process permitting spatiotemporally resolved cellular response that is also capable of learning complex nonlinear dynamical cellular behavior. We demonstrate the proposed ion dynamics permits rapid dissemination of response to information extrinsic perturbations that is consistent with experimental observations.
Precision Radiotherapy via Information Integration of Expert Human Knowledge and AI Recommendation to Optimize Clinical Decision Making
Feb 09, 2022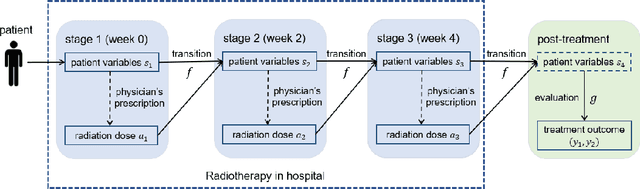
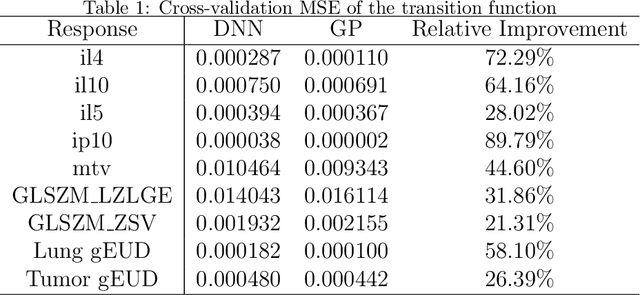
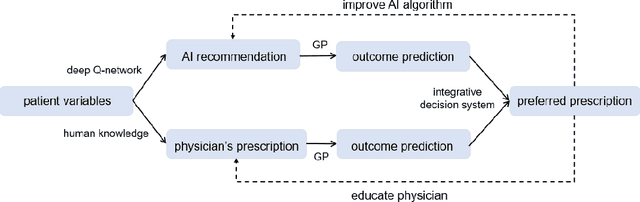
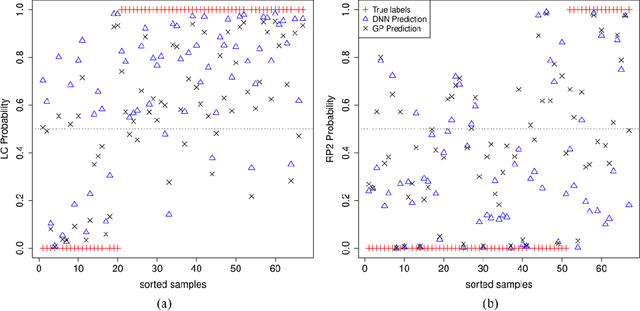
Abstract:In the precision medicine era, there is a growing need for precision radiotherapy where the planned radiation dose needs to be optimally determined by considering a myriad of patient-specific information in order to ensure treatment efficacy. Existing artificial-intelligence (AI) methods can recommend radiation dose prescriptions within the scope of this available information. However, treating physicians may not fully entrust the AI's recommended prescriptions due to known limitations or when the AI recommendation may go beyond physicians' current knowledge. This paper lays out a systematic method to integrate expert human knowledge with AI recommendations for optimizing clinical decision making. Towards this goal, Gaussian process (GP) models are integrated with deep neural networks (DNNs) to quantify the uncertainty of the treatment outcomes given by physicians and AI recommendations, respectively, which are further used as a guideline to educate clinical physicians and improve AI models performance. The proposed method is demonstrated in a comprehensive dataset where patient-specific information and treatment outcomes are prospectively collected during radiotherapy of $67$ non-small cell lung cancer patients and retrospectively analyzed.
Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer
Mar 24, 2017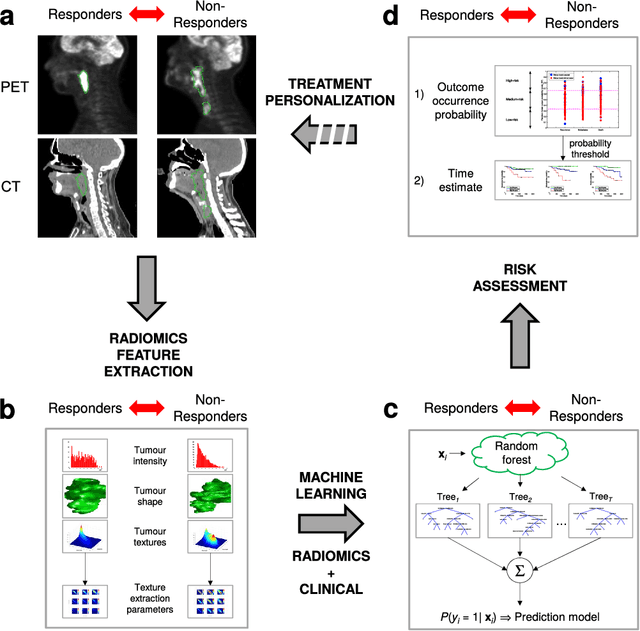
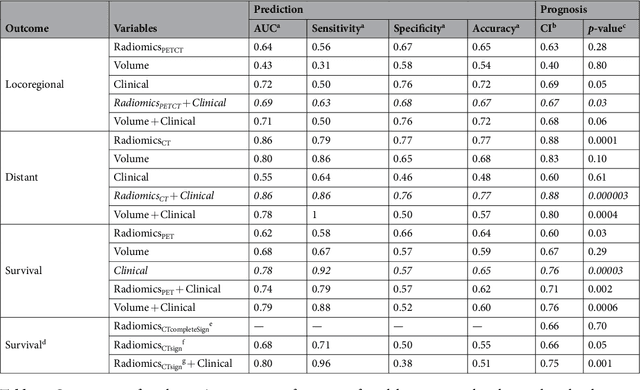
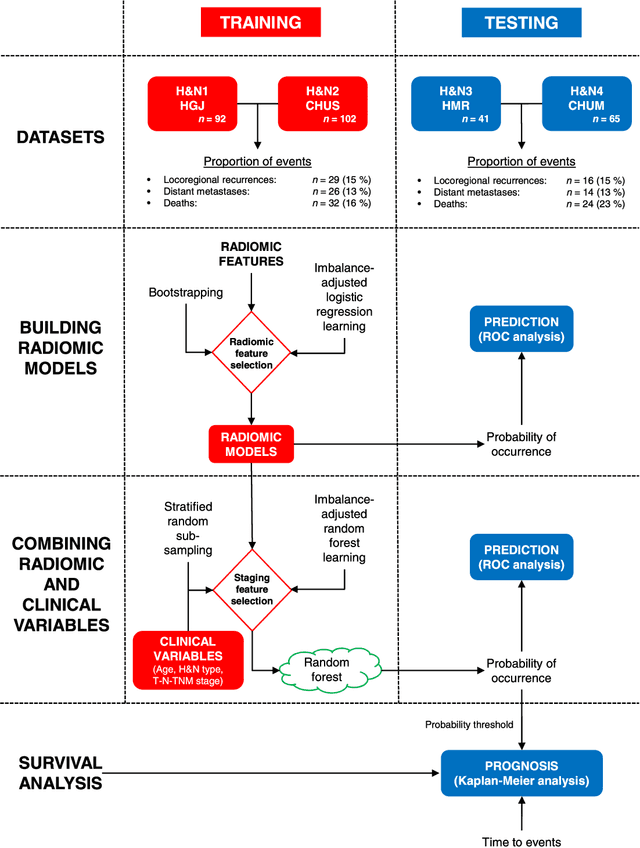
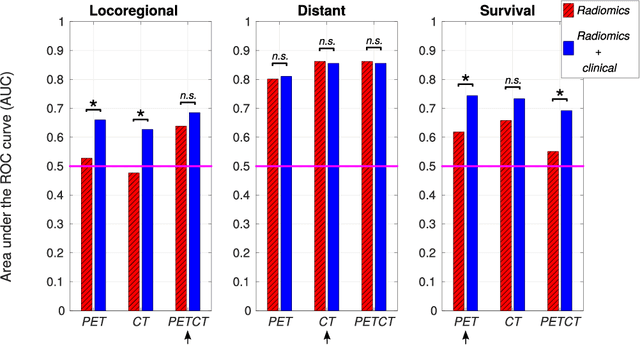
Abstract:Quantitative extraction of high-dimensional mineable data from medical images is a process known as radiomics. Radiomics is foreseen as an essential prognostic tool for cancer risk assessment and the quantification of intratumoural heterogeneity. In this work, 1615 radiomic features (quantifying tumour image intensity, shape, texture) extracted from pre-treatment FDG-PET and CT images of 300 patients from four different cohorts were analyzed for the risk assessment of locoregional recurrences (LR) and distant metastases (DM) in head-and-neck cancer. Prediction models combining radiomic and clinical variables were constructed via random forests and imbalance-adjustment strategies using two of the four cohorts. Independent validation of the prediction and prognostic performance of the models was carried out on the other two cohorts (LR: AUC = 0.69 and CI = 0.67; DM: AUC = 0.86 and CI = 0.88). Furthermore, the results obtained via Kaplan-Meier analysis demonstrated the potential of radiomics for assessing the risk of specific tumour outcomes using multiple stratification groups. This could have important clinical impact, notably by allowing for a better personalization of chemo-radiation treatments for head-and-neck cancer patients from different risk groups.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge