Dipesh Niraula
Department of Machine Learning, Moffitt Cancer Center, Tampa, FL, USA
Modeling non-genetic information dynamics in cells using reservoir computing
Dec 13, 2023Abstract:Virtually all cells use energy and ion-specific membrane pumps to maintain large transmembrane gradients of Na$^+$, K$^+$, Cl$^-$, Mg$^{++}$, and Ca$^{++}$. Although they consume up to 1/3 of a cell's energy budget, the corresponding evolutionary benefit of transmembrane ion gradients remain unclear. Here, we propose that ion gradients enable a dynamic and versatile biological system that acquires, analyzes, and responds to environmental information. We hypothesize environmental signals are transmitted into the cell by ion fluxes along pre-existing gradients through gated ion-specific membrane channels. The consequent changes of cytoplasmic ion concentration can generate a local response and orchestrate global or regional responses through wire-like ion fluxes along pre-existing and self-assembling cytoskeleton to engage the endoplasmic reticulum, mitochondria, and nucleus. Here, we frame our hypothesis through a quasi-physical (Cell-Reservoir) model that treats intra-cellular ion-based information dynamics as a sub-cellular process permitting spatiotemporally resolved cellular response that is also capable of learning complex nonlinear dynamical cellular behavior. We demonstrate the proposed ion dynamics permits rapid dissemination of response to information extrinsic perturbations that is consistent with experimental observations.
Precision Radiotherapy via Information Integration of Expert Human Knowledge and AI Recommendation to Optimize Clinical Decision Making
Feb 09, 2022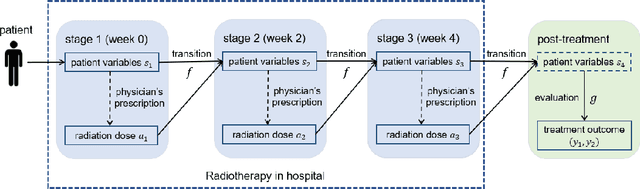
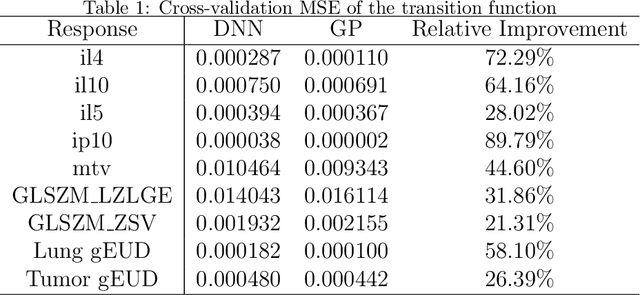
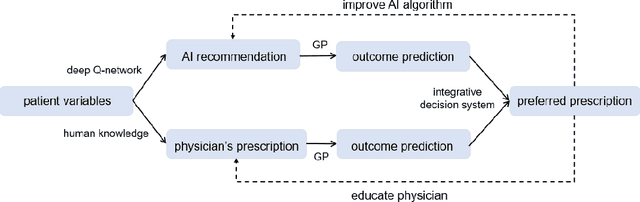
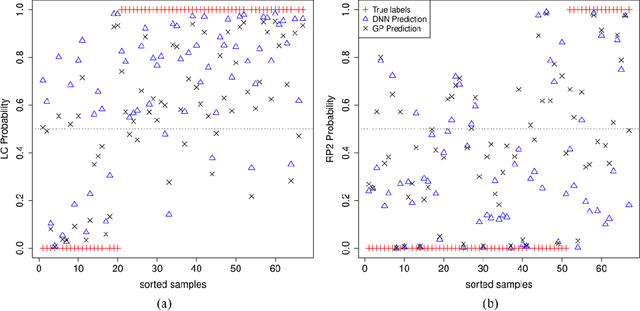
Abstract:In the precision medicine era, there is a growing need for precision radiotherapy where the planned radiation dose needs to be optimally determined by considering a myriad of patient-specific information in order to ensure treatment efficacy. Existing artificial-intelligence (AI) methods can recommend radiation dose prescriptions within the scope of this available information. However, treating physicians may not fully entrust the AI's recommended prescriptions due to known limitations or when the AI recommendation may go beyond physicians' current knowledge. This paper lays out a systematic method to integrate expert human knowledge with AI recommendations for optimizing clinical decision making. Towards this goal, Gaussian process (GP) models are integrated with deep neural networks (DNNs) to quantify the uncertainty of the treatment outcomes given by physicians and AI recommendations, respectively, which are further used as a guideline to educate clinical physicians and improve AI models performance. The proposed method is demonstrated in a comprehensive dataset where patient-specific information and treatment outcomes are prospectively collected during radiotherapy of $67$ non-small cell lung cancer patients and retrospectively analyzed.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge