Ilyess Zemmoura
Real-Time Brain Tumor Detection in Intraoperative Ultrasound Using YOLO11: From Model Training to Deployment in the Operating Room
Jan 27, 2025



Abstract:Intraoperative ultrasound (ioUS) is a valuable tool in brain tumor surgery due to its versatility, affordability, and seamless integration into the surgical workflow. However, its adoption remains limited, primarily because of the challenges associated with image interpretation and the steep learning curve required for effective use. This study aimed to enhance the interpretability of ioUS images by developing a real-time brain tumor detection system deployable in the operating room. We collected 2D ioUS images from the Brain Tumor Intraoperative Database (BraTioUS) and the public ReMIND dataset, annotated with expert-refined tumor labels. Using the YOLO11 architecture and its variants, we trained object detection models to identify brain tumors. The dataset included 1,732 images from 192 patients, divided into training, validation, and test sets. Data augmentation expanded the training set to 11,570 images. In the test dataset, YOLO11s achieved the best balance of precision and computational efficiency, with a mAP@50 of 0.95, mAP@50-95 of 0.65, and a processing speed of 34.16 frames per second. The proposed solution was prospectively validated in a cohort of 15 consecutively operated patients diagnosed with brain tumors. Neurosurgeons confirmed its seamless integration into the surgical workflow, with real-time predictions accurately delineating tumor regions. These findings highlight the potential of real-time object detection algorithms to enhance ioUS-guided brain tumor surgery, addressing key challenges in interpretation and providing a foundation for future development of computer vision-based tools for neuro-oncological surgery.
Joint Blind Deconvolution and Robust Principal Component Analysis for Blood Flow Estimation in Medical Ultrasound Imaging
Jul 10, 2020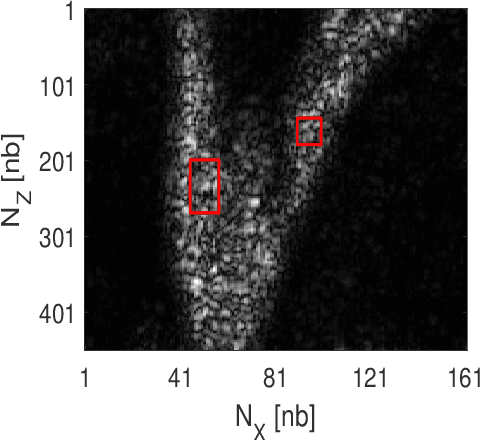
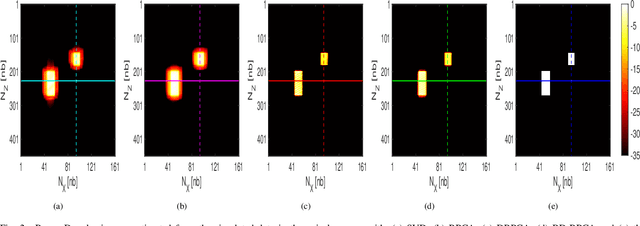
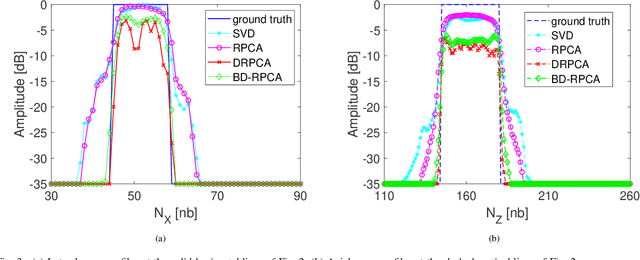
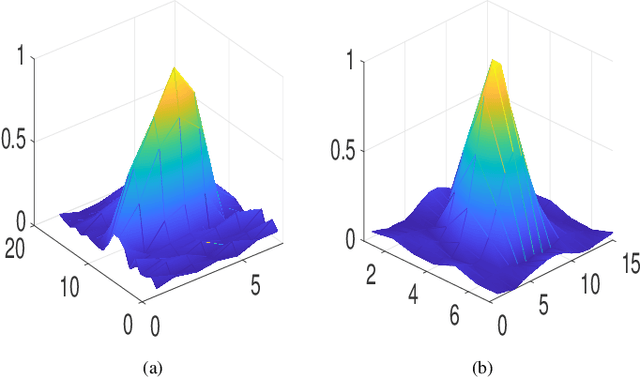
Abstract:This paper addresses the problem of high-resolution Doppler blood flow estimation from an ultrafast sequence of ultrasound images. Formulating the separation of clutter and blood components as an inverse problem has been shown in the literature to be a good alternative to spatio-temporal singular value decomposition (SVD)-based clutter filtering. In particular, a deconvolution step has recently been embedded in such a problem to mitigate the influence of the experimentally measured point spread function (PSF) of the imaging system. Deconvolution was shown in this context to improve the accuracy of the blood flow reconstruction. However, measuring the PSF requires non-trivial experimental setups. To overcome this limitation, we propose herein a blind deconvolution method able to estimate both the blood component and the PSF from Doppler data. Numerical experiments conducted on simulated and in vivo data demonstrate qualitatively and quantitatively the effectiveness of the proposed approach in comparison with the previous method based on experimentally measured PSF and two other state-of-the-art approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge