Iñigo Iturrate
Safe Uncertainty-Aware Learning of Robotic Suturing
May 22, 2025Abstract:Robot-Assisted Minimally Invasive Surgery is currently fully manually controlled by a trained surgeon. Automating this has great potential for alleviating issues, e.g., physical strain, highly repetitive tasks, and shortages of trained surgeons. For these reasons, recent works have utilized Artificial Intelligence methods, which show promising adaptability. Despite these advances, there is skepticism of these methods because they lack explainability and robust safety guarantees. This paper presents a framework for a safe, uncertainty-aware learning method. We train an Ensemble Model of Diffusion Policies using expert demonstrations of needle insertion. Using an Ensemble model, we can quantify the policy's epistemic uncertainty, which is used to determine Out-Of-Distribution scenarios. This allows the system to release control back to the surgeon in the event of an unsafe scenario. Additionally, we implement a model-free Control Barrier Function to place formal safety guarantees on the predicted action. We experimentally evaluate our proposed framework using a state-of-the-art robotic suturing simulator. We evaluate multiple scenarios, such as dropping the needle, moving the camera, and moving the phantom. The learned policy is robust to these perturbations, showing corrective behaviors and generalization, and it is possible to detect Out-Of-Distribution scenarios. We further demonstrate that the Control Barrier Function successfully limits the action to remain within our specified safety set in the case of unsafe predictions.
Safety-Ensured Control Framework for Robotic Endoscopic Task Automation
Mar 11, 2025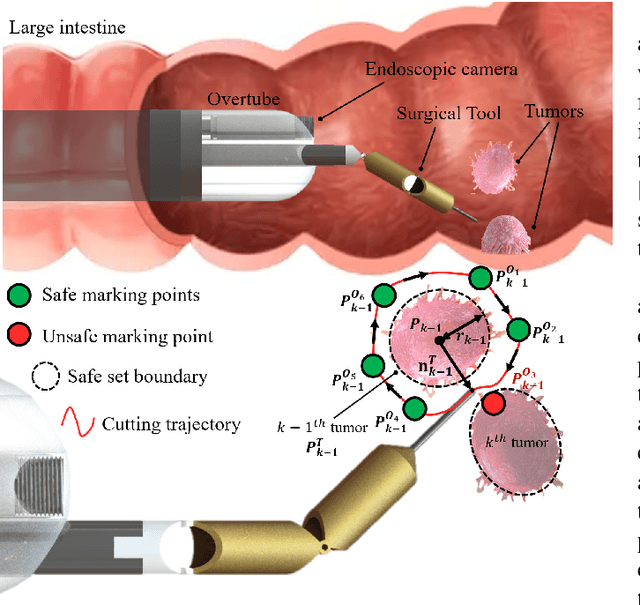
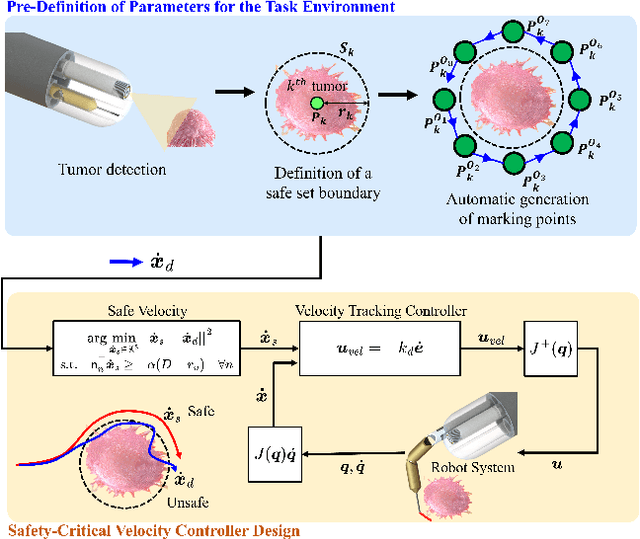
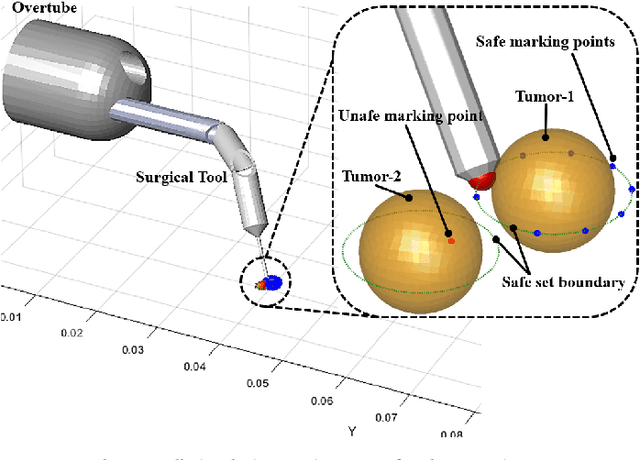
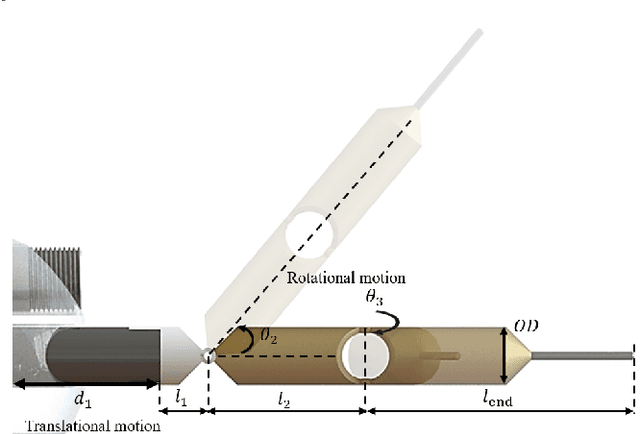
Abstract:There is growing interest in automating surgical tasks using robotic systems, such as endoscopy for treating gastrointestinal (GI) cancer. However, previous studies have primarily focused on detecting and analyzing objects or robots, with limited attention to ensuring safety, which is critical for clinical applications, where accidents can be caused by unsafe robot motions. In this study, we propose a new control framework that can formally ensure the safety of automating certain processes involved in endoscopic submucosal dissection (ESD), a representative endoscopic surgical method for the treatment of early GI cancer, by using an endoscopic robot. The proposed framework utilizes Control Barrier Functions (CBFs) to accurately identify the boundaries of individual tumors, even in close proximity within the GI tract, ensuring precise treatment and removal while preserving the surrounding normal tissue. Additionally, by adopting a model-free control scheme, safety assurance is made possible even in endoscopic robotic systems where dynamic modeling is challenging. We demonstrate the proposed framework in cases where the tumors to be removed are close to each other, showing that the safety constraints are enforced. We show that the model-free CBF-based controlled robot eliminates one tumor completely without damaging it, while not invading another nearby tumor.
Imitation Learning for Robotic Assisted Ultrasound Examination of Deep Venous Thrombosis using Kernelized Movement Primitives
Jul 11, 2024Abstract:Deep Vein Thrombosis (DVT) is a common yet potentially fatal condition, often leading to critical complications like pulmonary embolism. DVT is commonly diagnosed using Ultrasound (US) imaging, which can be inconsistent due to its high dependence on the operator's skill. Robotic US Systems (RUSs) aim to improve diagnostic test consistency but face challenges with the complex scanning pattern needed for DVT assessment, where precise control over US probe pressure is crucial for indirectly detecting occlusions. This work introduces an imitation learning method, based on Kernelized Movement Primitives (KMP), to standardize DVT US exams by training an autonomous robotic controller using sonographer demonstrations. A new recording device design enhances demonstration ergonomics, integrating with US probes and enabling seamless force and position data recording. KMPs are used to capture scanning skills, linking scan trajectory and force, enabling generalization beyond the demonstrations. Our approach, evaluated on synthetic models and volunteers, shows that the KMP-based RUS can replicate an expert's force control and image quality in DVT US examination. It outperforms previous methods using manually defined force profiles, improving exam standardization and reducing reliance on specialized sonographers.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge