Hasan Atakan Bedel
DreaMR: Diffusion-driven Counterfactual Explanation for Functional MRI
Jul 18, 2023Abstract:Deep learning analyses have offered sensitivity leaps in detection of cognitive states from functional MRI (fMRI) measurements across the brain. Yet, as deep models perform hierarchical nonlinear transformations on their input, interpreting the association between brain responses and cognitive states is challenging. Among common explanation approaches for deep fMRI classifiers, attribution methods show poor specificity and perturbation methods show limited plausibility. While counterfactual generation promises to address these limitations, previous methods use variational or adversarial priors that yield suboptimal sample fidelity. Here, we introduce the first diffusion-driven counterfactual method, DreaMR, to enable fMRI interpretation with high specificity, plausibility and fidelity. DreaMR performs diffusion-based resampling of an input fMRI sample to alter the decision of a downstream classifier, and then computes the minimal difference between the original and counterfactual samples for explanation. Unlike conventional diffusion methods, DreaMR leverages a novel fractional multi-phase-distilled diffusion prior to improve sampling efficiency without compromising fidelity, and it employs a transformer architecture to account for long-range spatiotemporal context in fMRI scans. Comprehensive experiments on neuroimaging datasets demonstrate the superior specificity, fidelity and efficiency of DreaMR in sample generation over state-of-the-art counterfactual methods for fMRI interpretation.
BolT: Fused Window Transformers for fMRI Time Series Analysis
May 23, 2022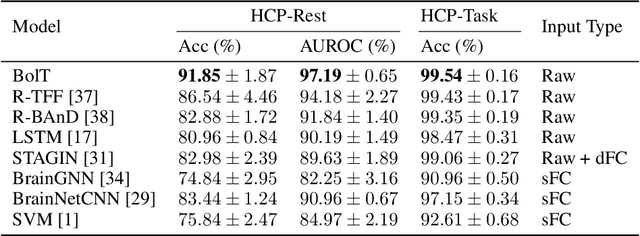
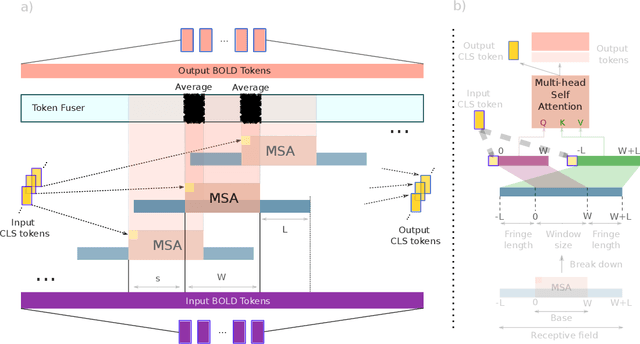
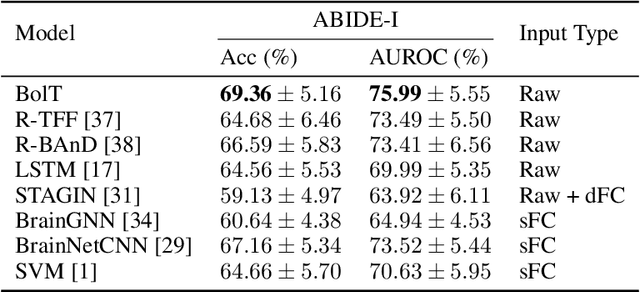
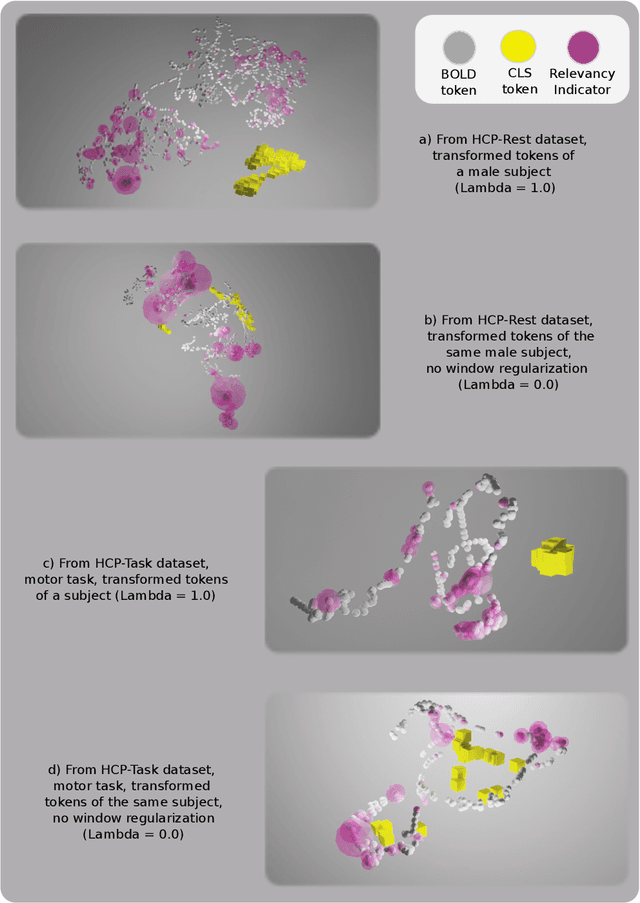
Abstract:Functional magnetic resonance imaging (fMRI) enables examination of inter-regional interactions in the brain via functional connectivity (FC) analyses that measure the synchrony between the temporal activations of separate regions. Given their exceptional sensitivity, deep-learning methods have received growing interest for FC analyses of high-dimensional fMRI data. In this domain, models that operate directly on raw time series as opposed to pre-computed FC features have the potential benefit of leveraging the full scale of information present in fMRI data. However, previous models are based on architectures suboptimal for temporal integration of representations across multiple time scales. Here, we present BolT, blood-oxygen-level-dependent transformer, for analyzing multi-variate fMRI time series. BolT leverages a cascade of transformer encoders equipped with a novel fused window attention mechanism. Transformer encoding is performed on temporally-overlapped time windows within the fMRI time series to capture short time-scale representations. To integrate information across windows, cross-window attention is computed between base tokens in each time window and fringe tokens from neighboring time windows. To transition from local to global representations, the extent of window overlap and thereby number of fringe tokens is progressively increased across the cascade. Finally, a novel cross-window regularization is enforced to align the high-level representations of global $CLS$ features across time windows. Comprehensive experiments on public fMRI datasets clearly illustrate the superior performance of BolT against state-of-the-art methods. Posthoc explanatory analyses to identify landmark time points and regions that contribute most significantly to model decisions corroborate prominent neuroscientific findings from recent fMRI studies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge