Harish RaviPrakash
Variational Capsule Encoder
Oct 18, 2020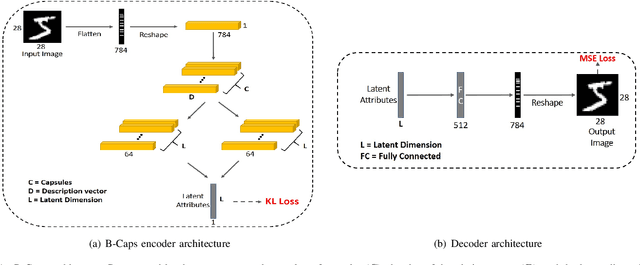
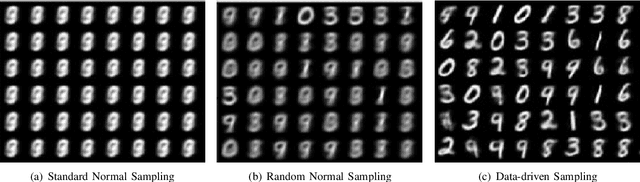
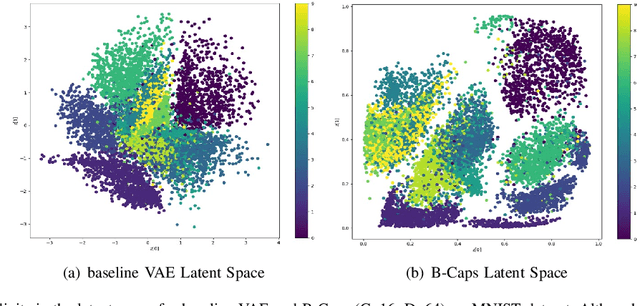

Abstract:We propose a novel capsule network based variational encoder architecture, called Bayesian capsules (B-Caps), to modulate the mean and standard deviation of the sampling distribution in the latent space. We hypothesized that this approach can learn a better representation of features in the latent space than traditional approaches. Our hypothesis was tested by using the learned latent variables for image reconstruction task, where for MNIST and Fashion-MNIST datasets, different classes were separated successfully in the latent space using our proposed model. Our experimental results have shown improved reconstruction and classification performances for both datasets adding credence to our hypothesis. We also showed that by increasing the latent space dimension, the proposed B-Caps was able to learn a better representation when compared to the traditional variational auto-encoders (VAE). Hence our results indicate the strength of capsule networks in representation learning which has never been examined under the VAE settings before.
Brain Tumor Survival Prediction using Radiomics Features
Sep 07, 2020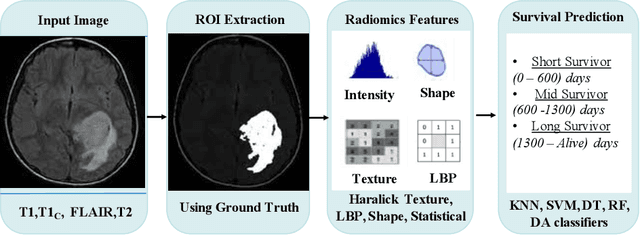



Abstract:Surgery planning in patients diagnosed with brain tumor is dependent on their survival prognosis. A poor prognosis might demand for a more aggressive treatment and therapy plan, while a favorable prognosis might enable a less risky surgery plan. Thus, accurate survival prognosis is an important step in treatment planning. Recently, deep learning approaches have been used extensively for brain tumor segmentation followed by the use of deep features for prognosis. However, radiomics-based studies have shown more promise using engineered/hand-crafted features. In this paper, we propose a three-step approach for multi-class survival prognosis. In the first stage, we extract image slices corresponding to tumor regions from multiple magnetic resonance image modalities. We then extract radiomic features from these 2D slices. Finally, we train machine learning classifiers to perform the classification. We evaluate our proposed approach on the publicly available BraTS 2019 data and achieve an accuracy of 76.5% and precision of 74.3% using the random forest classifier, which to the best of our knowledge are the highest reported results yet. Further, we identify the most important features that contribute in improving the prediction.
A Survey on Recent Advancements for AI Enabled Radiomics in Neuro-Oncology
Oct 16, 2019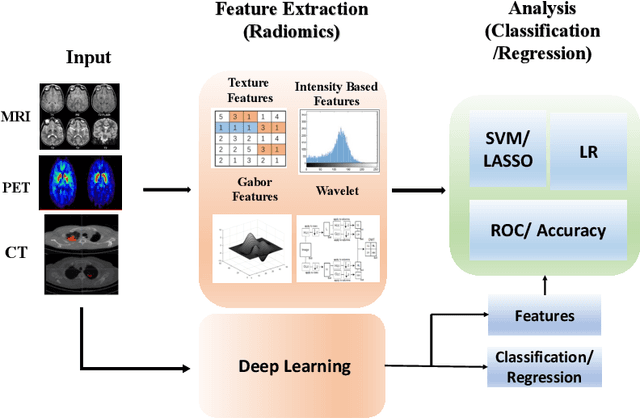
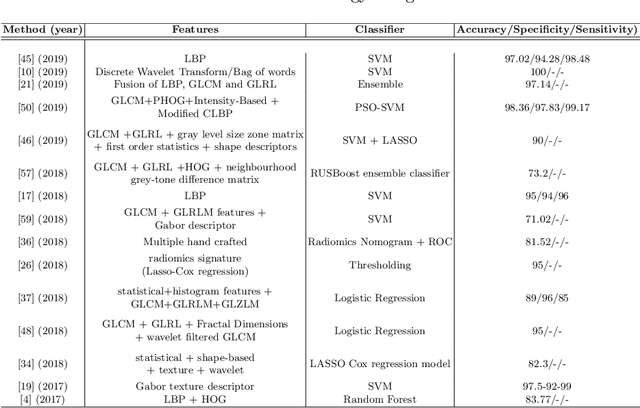
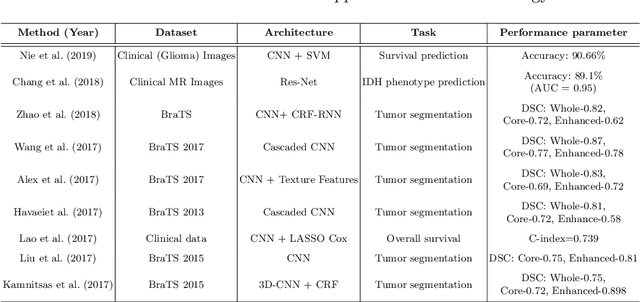
Abstract:Artificial intelligence (AI) enabled radiomics has evolved immensely especially in the field of oncology. Radiomics provide assistancein diagnosis of cancer, planning of treatment strategy, and predictionof survival. Radiomics in neuro-oncology has progressed significantly inthe recent past. Deep learning has outperformed conventional machinelearning methods in most image-based applications. Convolutional neu-ral networks (CNNs) have seen some popularity in radiomics, since theydo not require hand-crafted features and can automatically extract fea-tures during the learning process. In this regard, it is observed that CNNbased radiomics could provide state-of-the-art results in neuro-oncology,similar to the recent success of such methods in a wide spectrum ofmedical image analysis applications. Herein we present a review of the most recent best practices and establish the future trends for AI enabled radiomics in neuro-oncology.
Automatic Response Assessment in Regions of Language Cortex in Epilepsy Patients Using ECoG-based Functional Mapping and Machine Learning
Aug 06, 2017



Abstract:Accurate localization of brain regions responsible for language and cognitive functions in Epilepsy patients should be carefully determined prior to surgery. Electrocorticography (ECoG)-based Real Time Functional Mapping (RTFM) has been shown to be a safer alternative to the electrical cortical stimulation mapping (ESM), which is currently the clinical/gold standard. Conventional methods for analyzing RTFM signals are based on statistical comparison of signal power at certain frequency bands. Compared to gold standard (ESM), they have limited accuracies when assessing channel responses. In this study, we address the accuracy limitation of the current RTFM signal estimation methods by analyzing the full frequency spectrum of the signal and replacing signal power estimation methods with machine learning algorithms, specifically random forest (RF), as a proof of concept. We train RF with power spectral density of the time-series RTFM signal in supervised learning framework where ground truth labels are obtained from the ESM. Results obtained from RTFM of six adult patients in a strictly controlled experimental setup reveal the state of the art detection accuracy of $\approx 78\%$ for the language comprehension task, an improvement of $23\%$ over the conventional RTFM estimation method. To the best of our knowledge, this is the first study exploring the use of machine learning approaches for determining RTFM signal characteristics, and using the whole-frequency band for better region localization. Our results demonstrate the feasibility of machine learning based RTFM signal analysis method over the full spectrum to be a clinical routine in the near future.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge