Frank Rademakers
Comparison of static and dynamic random forests models for EHR data in the presence of competing risks: predicting central line-associated bloodstream infection
Apr 24, 2024



Abstract:Prognostic outcomes related to hospital admissions typically do not suffer from censoring, and can be modeled either categorically or as time-to-event. Competing events are common but often ignored. We compared the performance of random forest (RF) models to predict the risk of central line-associated bloodstream infections (CLABSI) using different outcome operationalizations. We included data from 27478 admissions to the University Hospitals Leuven, covering 30862 catheter episodes (970 CLABSI, 1466 deaths and 28426 discharges) to build static and dynamic RF models for binary (CLABSI vs no CLABSI), multinomial (CLABSI, discharge, death or no event), survival (time to CLABSI) and competing risks (time to CLABSI, discharge or death) outcomes to predict the 7-day CLABSI risk. We evaluated model performance across 100 train/test splits. Performance of binary, multinomial and competing risks models was similar: AUROC was 0.74 for baseline predictions, rose to 0.78 for predictions at day 5 in the catheter episode, and decreased thereafter. Survival models overestimated the risk of CLABSI (E:O ratios between 1.2 and 1.6), and had AUROCs about 0.01 lower than other models. Binary and multinomial models had lowest computation times. Models including multiple outcome events (multinomial and competing risks) display a different internal structure compared to binary and survival models. In the absence of censoring, complex modelling choices do not considerably improve the predictive performance compared to a binary model for CLABSI prediction in our studied settings. Survival models censoring the competing events at their time of occurrence should be avoided.
Length of Stay prediction for Hospital Management using Domain Adaptation
Jun 29, 2023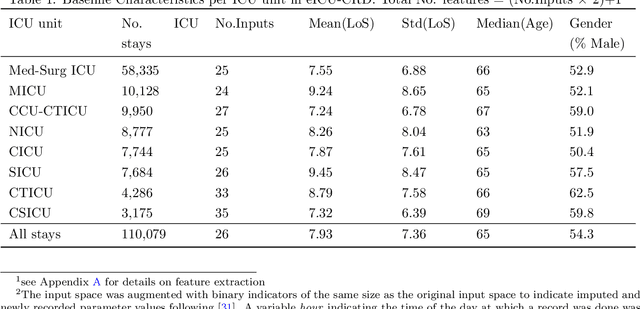
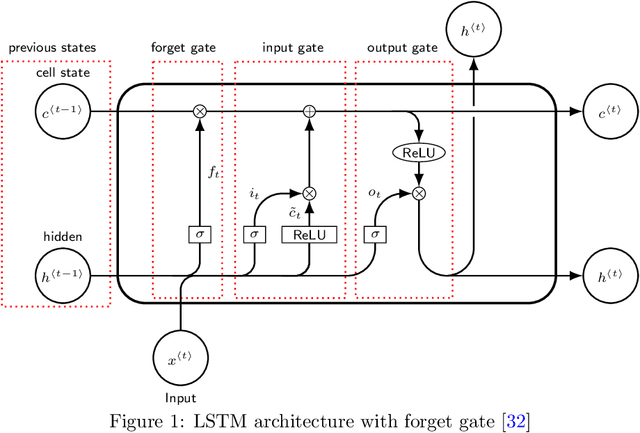
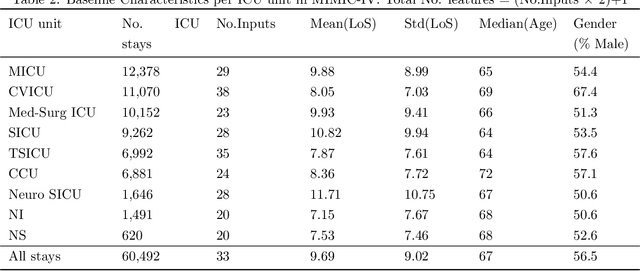
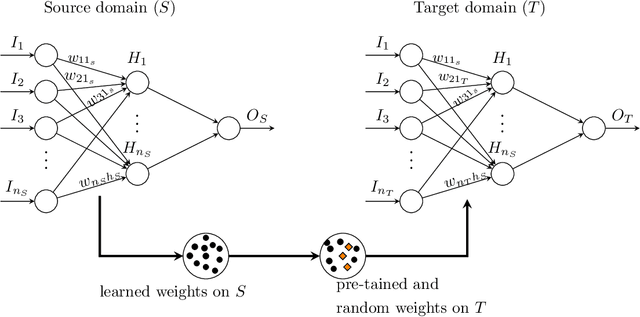
Abstract:Inpatient length of stay (LoS) is an important managerial metric which if known in advance can be used to efficiently plan admissions, allocate resources and improve care. Using historical patient data and machine learning techniques, LoS prediction models can be developed. Ethically, these models can not be used for patient discharge in lieu of unit heads but are of utmost necessity for hospital management systems in charge of effective hospital planning. Therefore, the design of the prediction system should be adapted to work in a true hospital setting. In this study, we predict early hospital LoS at the granular level of admission units by applying domain adaptation to leverage information learned from a potential source domain. Time-varying data from 110,079 and 60,492 patient stays to 8 and 9 intensive care units were respectively extracted from eICU-CRD and MIMIC-IV. These were fed into a Long-Short Term Memory and a Fully connected network to train a source domain model, the weights of which were transferred either partially or fully to initiate training in target domains. Shapley Additive exPlanations (SHAP) algorithms were used to study the effect of weight transfer on model explanability. Compared to the benchmark, the proposed weight transfer model showed statistically significant gains in prediction accuracy (between 1% and 5%) as well as computation time (up to 2hrs) for some target domains. The proposed method thus provides an adapted clinical decision support system for hospital management that can ease processes of data access via ethical committee, computation infrastructures and time.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge