Francesco Raimondi
Interpretability from a new lens: Integrating Stratification and Domain knowledge for Biomedical Applications
Mar 15, 2023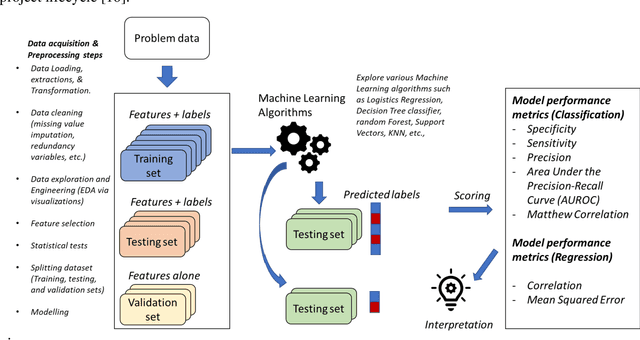
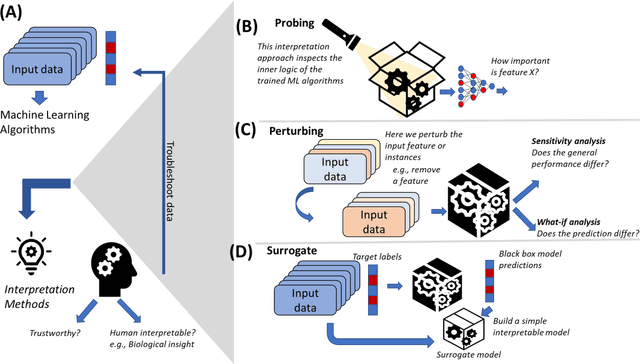
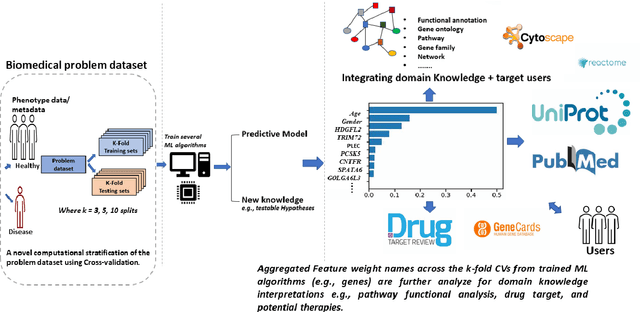
Abstract:The use of machine learning (ML) techniques in the biomedical field has become increasingly important, particularly with the large amounts of data generated by the aftermath of the COVID-19 pandemic. However, due to the complex nature of biomedical datasets and the use of black-box ML models, a lack of trust and adoption by domain experts can arise. In response, interpretable ML (IML) approaches have been developed, but the curse of dimensionality in biomedical datasets can lead to model instability. This paper proposes a novel computational strategy for the stratification of biomedical problem datasets into k-fold cross-validation (CVs) and integrating domain knowledge interpretation techniques embedded into the current state-of-the-art IML frameworks. This approach can improve model stability, establish trust, and provide explanations for outcomes generated by trained IML models. Specifically, the model outcome, such as aggregated feature weight importance, can be linked to further domain knowledge interpretations using techniques like pathway functional enrichment, drug targeting, and repurposing databases. Additionally, involving end-users and clinicians in focus group discussions before and after the choice of IML framework can help guide testable hypotheses, improve performance metrics, and build trustworthy and usable IML solutions in the biomedical field. Overall, this study highlights the potential of combining advanced computational techniques with domain knowledge interpretation to enhance the effectiveness of IML solutions in the context of complex biomedical datasets.
Deep learning in the ultrasound evaluation of neonatal respiratory status
Oct 31, 2020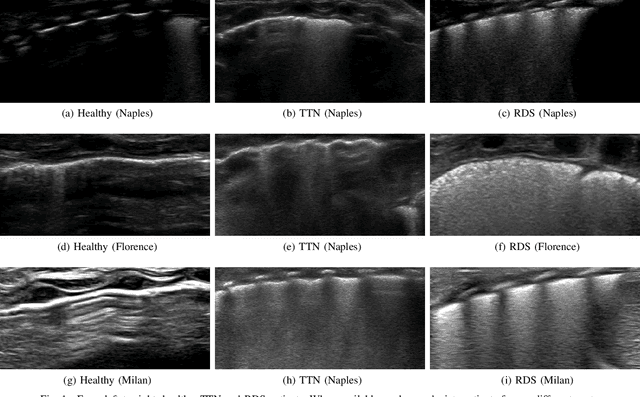
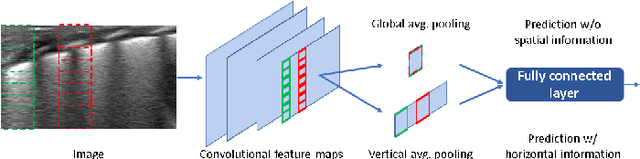
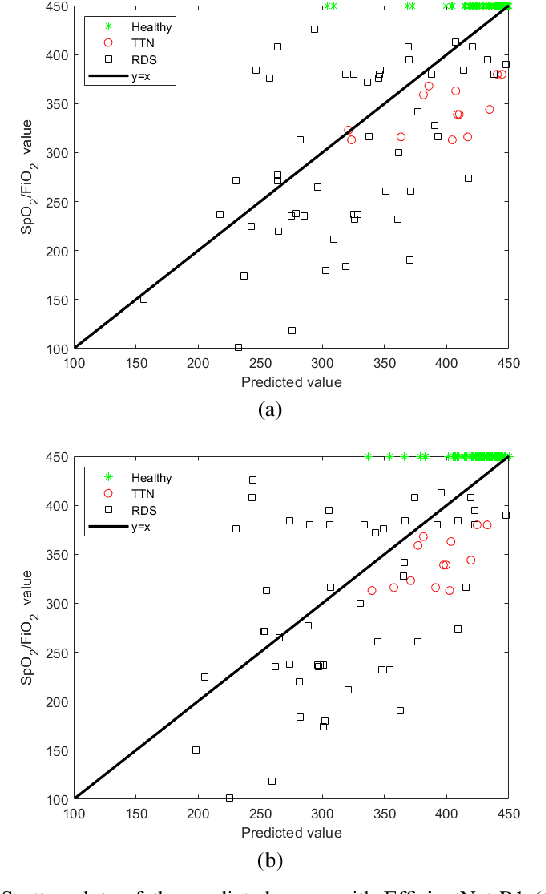
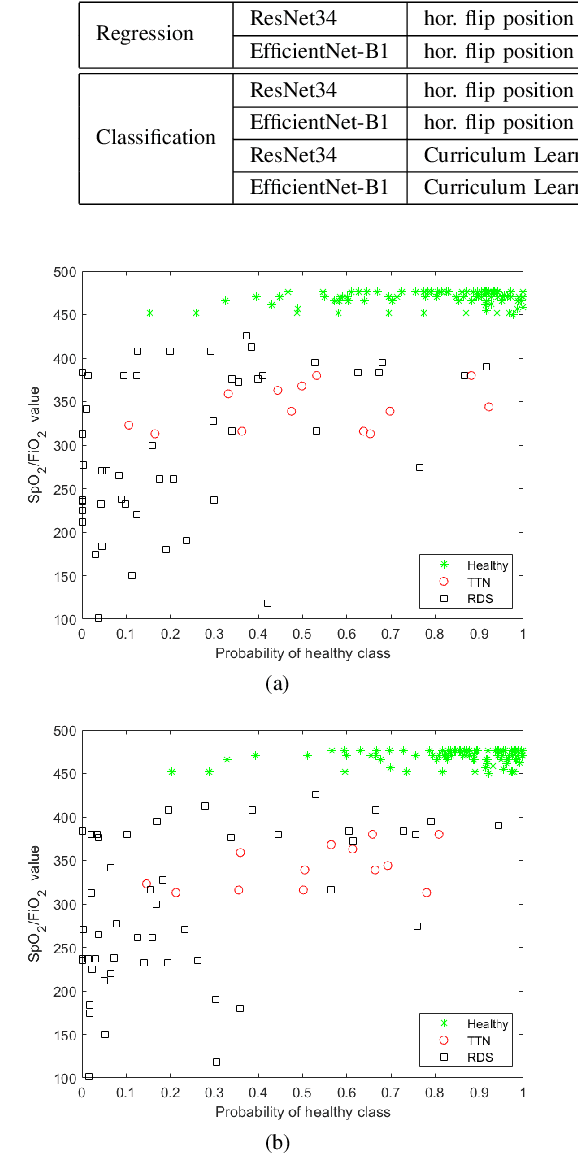
Abstract:Lung ultrasound imaging is reaching growing interest from the scientific community. On one side, thanks to its harmlessness and high descriptive power, this kind of diagnostic imaging has been largely adopted in sensitive applications, like the diagnosis and follow-up of preterm newborns in neonatal intensive care units. On the other side, state-of-the-art image analysis and pattern recognition approaches have recently proven their ability to fully exploit the rich information contained in these data, making them attractive for the research community. In this work, we present a thorough analysis of recent deep learning networks and training strategies carried out on a vast and challenging multicenter dataset comprising 87 patients with different diseases and gestational ages. These approaches are employed to assess the lung respiratory status from ultrasound images and are evaluated against a reference marker. The conducted analysis sheds some light on this problem by showing the critical points that can mislead the training procedure and proposes some adaptations to the specific data and task. The achieved results sensibly outperform those obtained by a previous work, which is based on textural features, and narrow the gap with the visual score predicted by the human experts.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge