Fatema Tuz Zohora
PointIso: Point Cloud Based Deep Learning Model for Detecting Arbitrary-Precision Peptide Features in LC-MS Map through Attention Based Segmentation
Sep 15, 2020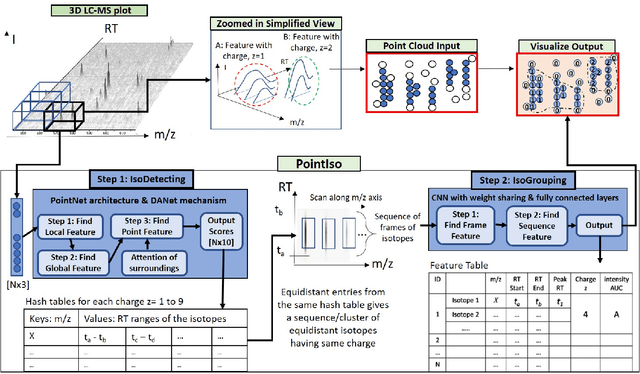


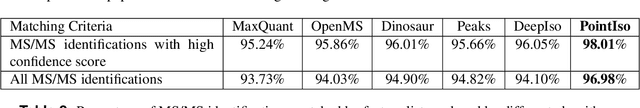
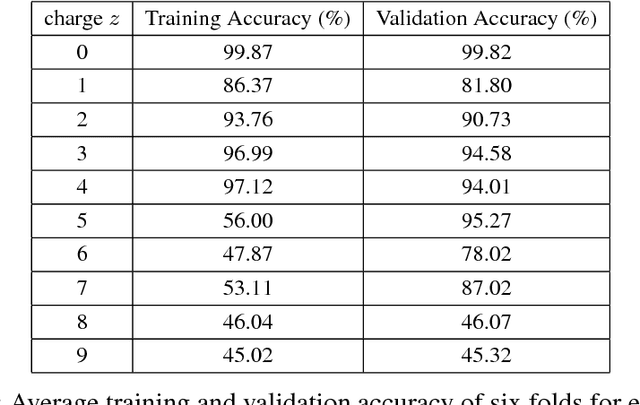
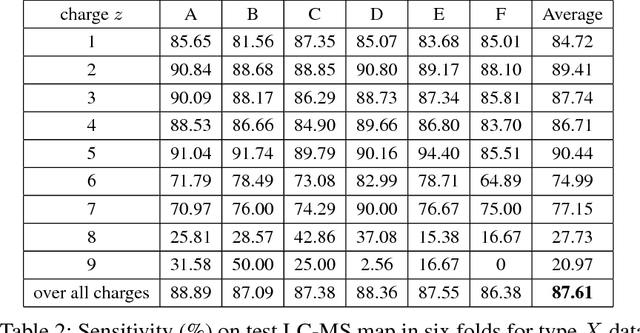
Abstract:A promising technique of discovering disease biomarkers is to measure the relative protein abundance in multiple biofluid samples through liquid chromatography with tandem mass spectrometry (LC-MS/MS) based quantitative proteomics. The key step involves peptide feature detection in LC-MS map, along with its charge and intensity. Existing heuristic algorithms suffer from inaccurate parameters since different settings of the parameters result in significantly different outcomes. Therefore, we propose PointIso, to serve the necessity of an automated system for peptide feature detection that is able to find out the proper parameters itself, and is easily adaptable to different types of datasets. It consists of an attention based scanning step for segmenting the multi-isotopic pattern of peptide features along with charge and a sequence classification step for grouping those isotopes into potential peptide features. PointIso is the first point cloud based, arbitrary-precision deep learning network to address the problem and achieves 98% detection of high quality MS/MS identifications in a benchmark dataset, which is higher than several other widely used algorithms. Besides contributing to the proteomics study, we believe our novel segmentation technique should serve the general image processing domain as well.
DeepIso: A Deep Learning Model for Peptide Feature Detection
Dec 09, 2017

Abstract:Liquid chromatography with tandem mass spectrometry (LC-MS/MS) based proteomics is a well-established research field with major applications such as identification of disease biomarkers, drug discovery, drug design and development. In proteomics, protein identification and quantification is a fundamental task, which is done by first enzymatically digesting it into peptides, and then analyzing peptides by LC-MS/MS instruments. The peptide feature detection and quantification from an LC-MS map is the first step in typical analysis workflows. In this paper we propose a novel deep learning based model, DeepIso, that uses Convolutional Neural Networks (CNNs) to scan an LC-MS map to detect peptide features and estimate their abundance. Existing tools are often designed with limited engineered features based on domain knowledge, and depend on pretrained parameters which are hardly updated despite huge amount of new coming proteomic data. Our proposed model, on the other hand, is capable of learning multiple levels of representation of high dimensional data through its many layers of neurons and continuously evolving with newly acquired data. To evaluate our proposed model, we use an antibody dataset including a heavy and a light chain, each digested by Asp-N, Chymotrypsin, Trypsin, thus giving six LC-MS maps for the experiment. Our model achieves 93.21% sensitivity with specificity of 99.44% on this dataset. Our results demonstrate that novel deep learning tools are desirable to advance the state-of-the-art in protein identification and quantification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge