David Benrimoh
LLM-Assisted Abstract Screening with OLIVER: Evaluating Calibration and Single-Model vs. Actor-Critic Configurations in Literature Reviews
Dec 23, 2025Abstract:Introduction: Recent work suggests large language models (LLMs) can accelerate screening, but prior evaluations focus on earlier LLMs, standardized Cochrane reviews, single-model setups, and accuracy as the primary metric, leaving generalizability, configuration effects, and calibration largely unexamined. Methods: We developed OLIVER (Optimized LLM-based Inclusion and Vetting Engine for Reviews), an open-source pipeline for LLM-assisted abstract screening. We evaluated multiple contemporary LLMs across two non-Cochrane systematic reviews and performance was assessed at both the full-text screening and final inclusion stages using accuracy, AUC, and calibration metrics. We further tested an actor-critic screening framework combining two lightweight models under three aggregation rules. Results: Across individual models, performance varied widely. In the smaller Review 1 (821 abstracts, 63 final includes), several models achieved high sensitivity for final includes but at the cost of substantial false positives and poor calibration. In the larger Review 2 (7741 abstracts, 71 final includes), most models were highly specific but struggled to recover true includes, with prompt design influencing recall. Calibration was consistently weak across single-model configurations despite high overall accuracy. Actor-critic screening improved discrimination and markedly reduced calibration error in both reviews, yielding higher AUCs. Discussion: LLMs may eventually accelerate abstract screening, but single-model performance is highly sensitive to review characteristics, prompting, and calibration is limited. An actor-critic framework improves classification quality and confidence reliability while remaining computationally efficient, enabling large-scale screening at low cost.
The Einstein Test: Towards a Practical Test of a Machine's Ability to Exhibit Superintelligence
Jan 12, 2025Abstract:Creative and disruptive insights (CDIs), such as the development of the theory of relativity, have punctuated human history, marking pivotal shifts in our intellectual trajectory. Recent advancements in artificial intelligence (AI) have sparked debates over whether state of the art models possess the capacity to generate CDIs. We argue that the ability to create CDIs should be regarded as a significant feature of machine superintelligence (SI).To this end, we propose a practical test to evaluate whether an approach to AI targeting SI can yield novel insights of this kind. We propose the Einstein test: given the data available prior to the emergence of a known CDI, can an AI independently reproduce that insight (or one that is formally equivalent)? By achieving such a milestone, a machine can be considered to at least match humanity's past top intellectual achievements, and therefore to have the potential to surpass them.
Towards Outcome-Driven Patient Subgroups: A Machine Learning Analysis Across Six Depression Treatment Studies
Mar 30, 2023Abstract:Major depressive disorder (MDD) is a heterogeneous condition; multiple underlying neurobiological substrates could be associated with treatment response variability. Understanding the sources of this variability and predicting outcomes has been elusive. Machine learning has shown promise in predicting treatment response in MDD, but one limitation has been the lack of clinical interpretability of machine learning models. We analyzed data from six clinical trials of pharmacological treatment for depression (total n = 5438) using the Differential Prototypes Neural Network (DPNN), a neural network model that derives patient prototypes which can be used to derive treatment-relevant patient clusters while learning to generate probabilities for differential treatment response. A model classifying remission and outputting individual remission probabilities for five first-line monotherapies and three combination treatments was trained using clinical and demographic data. Model validity and clinical utility were measured based on area under the curve (AUC) and expected improvement in sample remission rate with model-guided treatment, respectively. Post-hoc analyses yielded clusters (subgroups) based on patient prototypes learned during training. Prototypes were evaluated for interpretability by assessing differences in feature distributions and treatment-specific outcomes. A 3-prototype model achieved an AUC of 0.66 and an expected absolute improvement in population remission rate compared to the sample remission rate. We identified three treatment-relevant patient clusters which were clinically interpretable. It is possible to produce novel treatment-relevant patient profiles using machine learning models; doing so may improve precision medicine for depression. Note: This model is not currently the subject of any active clinical trials and is not intended for clinical use.
Applying Artificial Intelligence to Clinical Decision Support in Mental Health: What Have We Learned?
Mar 06, 2023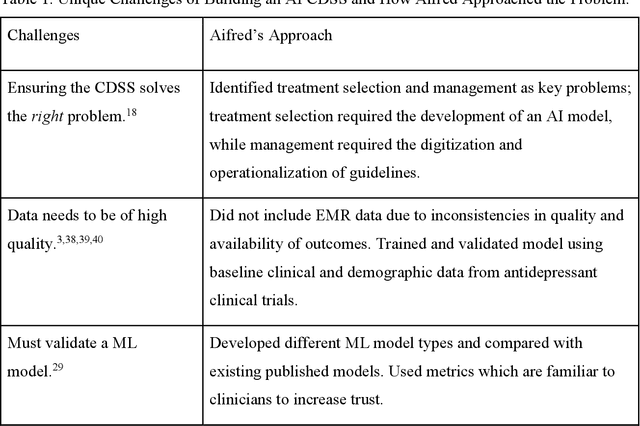
Abstract:Clinical decision support systems (CDSS) augmented with artificial intelligence (AI) models are emerging as potentially valuable tools in healthcare. Despite their promise, the development and implementation of these systems typically encounter several barriers, hindering the potential for widespread adoption. Here we present a case study of a recently developed AI-CDSS, Aifred Health, aimed at supporting the selection and management of treatment in major depressive disorder. We consider both the principles espoused during development and testing of this AI-CDSS, as well as the practical solutions developed to facilitate implementation. We also propose recommendations to consider throughout the building, validation, training, and implementation process of an AI-CDSS. These recommendations include: identifying the key problem, selecting the type of machine learning approach based on this problem, determining the type of data required, determining the format required for a CDSS to provide clinical utility, gathering physician and patient feedback, and validating the tool across multiple settings. Finally, we explore the potential benefits of widespread adoption of these systems, while balancing these against implementation challenges such as ensuring systems do not disrupt the clinical workflow, and designing systems in a manner that engenders trust on the part of end users.
Computational Mechanism for the Effect of Psychosis Community Treatment: A Conceptual Review from Neurobiology to Social Interaction
Mar 25, 2021


Abstract:The computational underpinnings of positive psychotic symptoms have recently received significant attention. Candidate mechanisms include some combination of maladaptive priors and reduced updating of these priors during perception. A potential benefit of models with such mechanisms is their ability to link multiple levels of explanation. This is key to improving how we understand the experience of psychosis. Moreover, it points us towards more comprehensive avenues for therapeutic research by providing a putative mechanism that could allow for the generation of new treatments from first principles. In order to demonstrate this, our conceptual paper will discuss the application of the insights from previous computational models to an important and complex set of evidence-based clinical interventions with strong social elements, such as coordinated specialty care clinics in early psychosis and assertive community treatment. These interventions may include but also go beyond psychopharmacology, providing, we argue, structure and predictability for patients experiencing psychosis. We develop the argument that this structure and predictability directly counteract the relatively low precision afforded to sensory information in psychosis, while also providing the patient more access to external cognitive resources in the form of providers and the structure of the programs themselves. We discuss how computational models explain the resulting reduction in symptoms, as well as the predictions these models make about potential responses of patients to modifications or to different variations of these interventions. We also link, via the framework of computational models, the experiences of patients and response to interventions to putative neurobiology.
Big Data Analytics and AI in Mental Healthcare
Mar 12, 2019Abstract:Mental health conditions cause a great deal of distress or impairment; depression alone will affect 11% of the world's population. The application of Artificial Intelligence (AI) and big-data technologies to mental health has great potential for personalizing treatment selection, prognosticating, monitoring for relapse, detecting and helping to prevent mental health conditions before they reach clinical-level symptomatology, and even delivering some treatments. However, unlike similar applications in other fields of medicine, there are several unique challenges in mental health applications which currently pose barriers towards the implementation of these technologies. Specifically, there are very few widely used or validated biomarkers in mental health, leading to a heavy reliance on patient and clinician derived questionnaire data as well as interpretation of new signals such as digital phenotyping. In addition, diagnosis also lacks the same objective 'gold standard' as in other conditions such as oncology, where clinicians and researchers can often rely on pathological analysis for confirmation of diagnosis. In this chapter we discuss the major opportunities, limitations and techniques used for improving mental healthcare through AI and big-data. We explore both the computational, clinical and ethical considerations and best practices as well as lay out the major researcher directions for the near future.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge