Daniel S Reich
Cortical lesions, central vein sign, and paramagnetic rim lesions in multiple sclerosis: emerging machine learning techniques and future avenues
Jan 19, 2022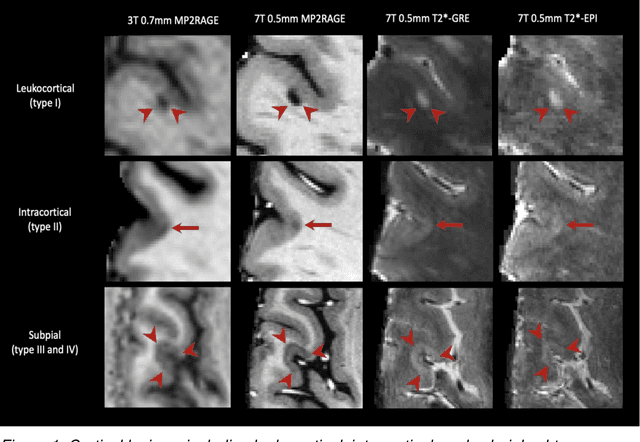
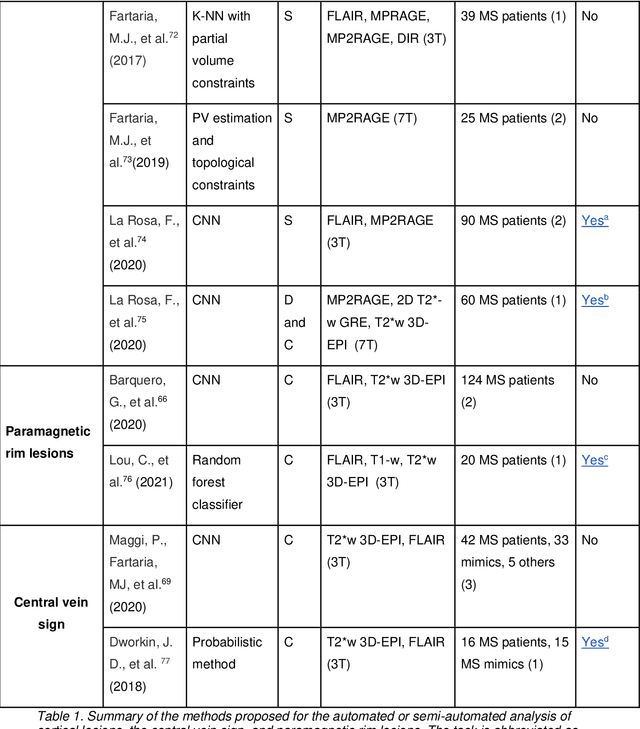
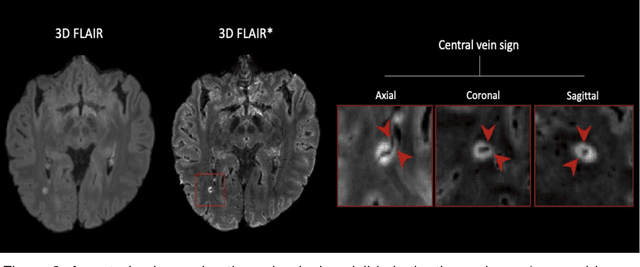
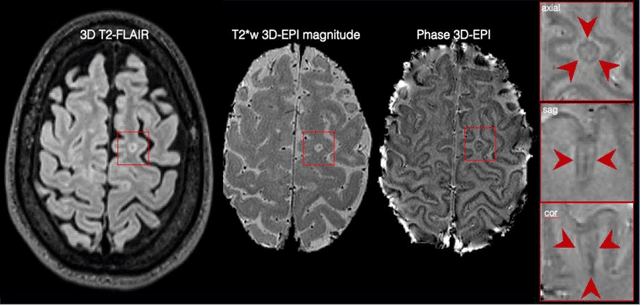
Abstract:The current multiple sclerosis (MS) diagnostic criteria lack specificity, and this may lead to misdiagnosis, which remains an issue in present-day clinical practice. In addition, conventional biomarkers only moderately correlate with MS disease progression. Recently, advanced MS lesional imaging biomarkers such as cortical lesions (CL), the central vein sign (CVS), and paramagnetic rim lesions (PRL), visible in specialized magnetic resonance imaging (MRI) sequences, have shown higher specificity in differential diagnosis. Moreover, studies have shown that CL and PRL are potential prognostic biomarkers, the former correlating with cognitive impairments and the latter with early disability progression. As machine learning-based methods have achieved extraordinary performance in the assessment of conventional imaging biomarkers, such as white matter lesion segmentation, several automated or semi-automated methods have been proposed for CL, CVS, and PRL as well. In the present review, we first introduce these advanced MS imaging biomarkers and their imaging methods. Subsequently, we describe the corresponding machine learning-based methods that were used to tackle these clinical questions, putting them into context with respect to the challenges they are still facing, including non-standardized MRI protocols, limited datasets, and moderate inter-rater variability. We conclude by presenting the current limitations that prevent their broader deployment and suggesting future research directions.
Automated Detection of Cortical Lesions in Multiple Sclerosis Patients with 7T MRI
Aug 15, 2020
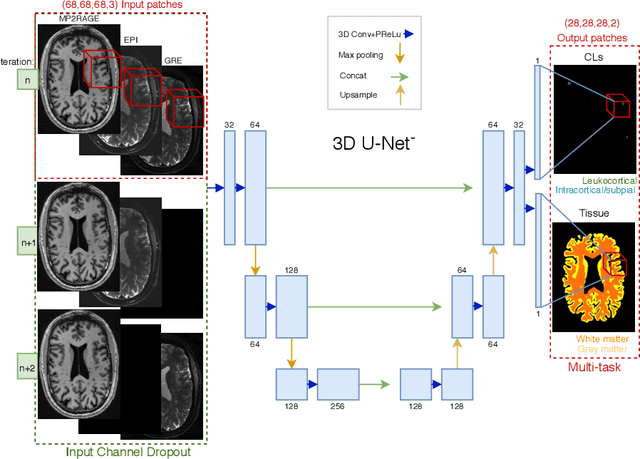

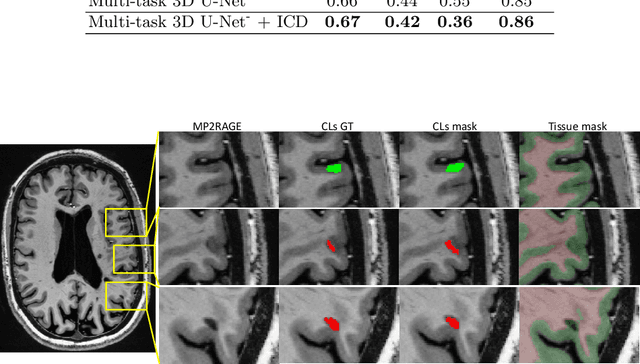
Abstract:The automated detection of cortical lesions (CLs) in patients with multiple sclerosis (MS) is a challenging task that, despite its clinical relevance, has received very little attention. Accurate detection of the small and scarce lesions requires specialized sequences and high or ultra-high field MRI. For supervised training based on multimodal structural MRI at 7T, two experts generated ground truth segmentation masks of 60 patients with 2014 CLs. We implemented a simplified 3D U-Net with three resolution levels (3D U-Net-). By increasing the complexity of the task (adding brain tissue segmentation), while randomly dropping input channels during training, we improved the performance compared to the baseline. Considering a minimum lesion size of 0.75 {\mu}L, we achieved a lesion-wise cortical lesion detection rate of 67% and a false positive rate of 42%. However, 393 (24%) of the lesions reported as false positives were post-hoc confirmed as potential or definite lesions by an expert. This indicates the potential of the proposed method to support experts in the tedious process of CL manual segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge