Ciro Franzese
Segmentation of Planning Target Volume in CT Series for Total Marrow Irradiation Using U-Net
Apr 05, 2023



Abstract:Radiotherapy (RT) is a key component in the treatment of various cancers, including Acute Lymphocytic Leukemia (ALL) and Acute Myelogenous Leukemia (AML). Precise delineation of organs at risk (OARs) and target areas is essential for effective treatment planning. Intensity Modulated Radiotherapy (IMRT) techniques, such as Total Marrow Irradiation (TMI) and Total Marrow and Lymph node Irradiation (TMLI), provide more precise radiation delivery compared to Total Body Irradiation (TBI). However, these techniques require time-consuming manual segmentation of structures in Computerized Tomography (CT) scans by the Radiation Oncologist (RO). In this paper, we present a deep learning-based auto-contouring method for segmenting Planning Target Volume (PTV) for TMLI treatment using the U-Net architecture. We trained and compared two segmentation models with two different loss functions on a dataset of 100 patients treated with TMLI at the Humanitas Research Hospital between 2011 and 2021. Despite challenges in lymph node areas, the best model achieved an average Dice score of 0.816 for PTV segmentation. Our findings are a preliminary but significant step towards developing a segmentation model that has the potential to save radiation oncologists a considerable amount of time. This could allow for the treatment of more patients, resulting in improved clinical practice efficiency and more reproducible contours.
Ensemble Methods for Multi-Organ Segmentation in CT Series
Mar 31, 2023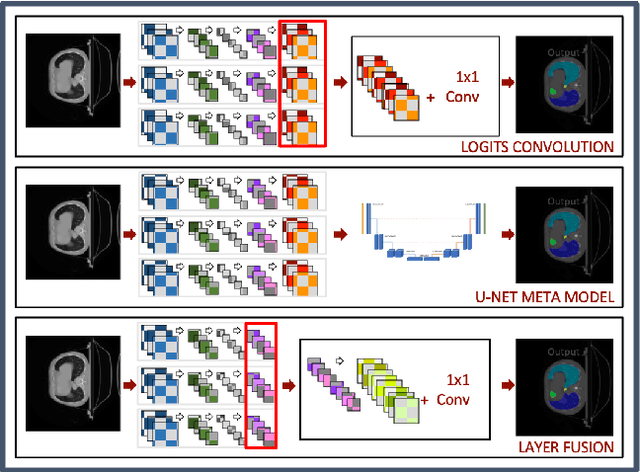
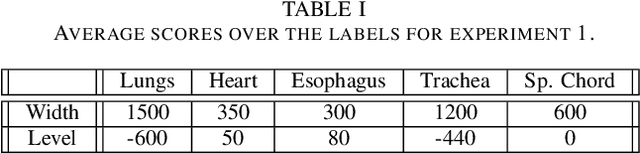
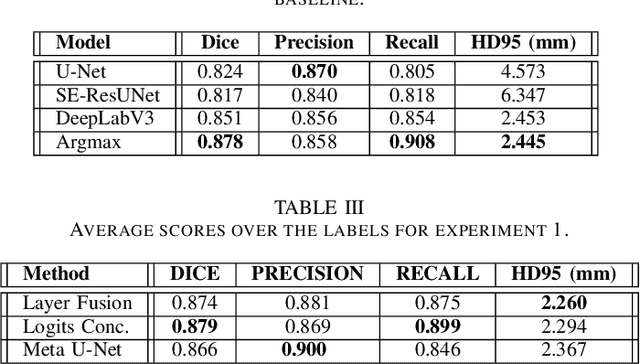
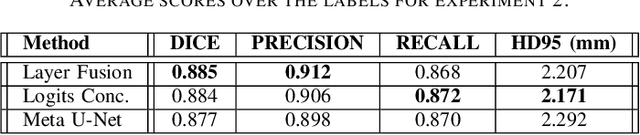
Abstract:In the medical images field, semantic segmentation is one of the most important, yet difficult and time-consuming tasks to be performed by physicians. Thanks to the recent advancement in the Deep Learning models regarding Computer Vision, the promise to automate this kind of task is getting more and more realistic. However, many problems are still to be solved, like the scarce availability of data and the difficulty to extend the efficiency of highly specialised models to general scenarios. Organs at risk segmentation for radiotherapy treatment planning falls in this category, as the limited data available negatively affects the possibility to develop general-purpose models; in this work, we focus on the possibility to solve this problem by presenting three types of ensembles of single-organ models able to produce multi-organ masks exploiting the different specialisations of their components. The results obtained are promising and prove that this is a possible solution to finding efficient multi-organ segmentation methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge