Bingqi Guo
Deep Learning-Based Automatic Delineation of Liver Domes in kV Triggered Images for Online Breath-hold Reproducibility Verification of Liver Stereotactic Body Radiation Therapy
Nov 22, 2024



Abstract:Stereotactic Body Radiation Therapy (SBRT) can be a precise, minimally invasive treatment method for liver cancer and liver metastases. However, the effectiveness of SBRT relies on the accurate delivery of the dose to the tumor while sparing healthy tissue. Challenges persist in ensuring breath-hold reproducibility, with current methods often requiring manual verification of liver dome positions from kV-triggered images. To address this, we propose a proof-of-principle study of a deep learning-based pipeline to automatically delineate the liver dome from kV-planar images. From 24 patients who received SBRT for liver cancer or metastasis inside liver, 711 KV-triggered images acquired for online breath-hold verification were included in the current study. We developed a pipeline comprising a trained U-Net for automatic liver dome region segmentation from the triggered images followed by extraction of the liver dome via thresholding, edge detection, and morphological operations. The performance and generalizability of the pipeline was evaluated using 2-fold cross validation. The training of the U-Net model for liver region segmentation took under 30 minutes and the automatic delineation of a liver dome for any triggered image took less than one second. The RMSE and rate of detection for Fold1 with 366 images was (6.4 +/- 1.6) mm and 91.7%, respectively. For Fold2 with 345 images, the RMSE and rate of detection was (7.7 +/- 2.3) mm and 76.3% respectively.
OpenKBP-Opt: An international and reproducible evaluation of 76 knowledge-based planning pipelines
Feb 16, 2022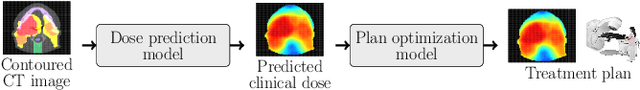

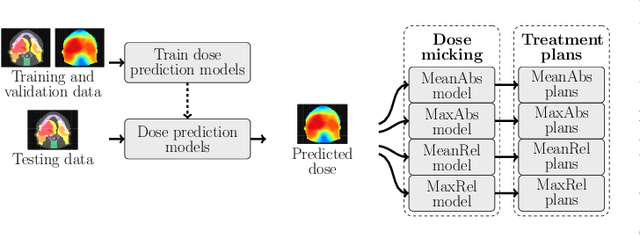
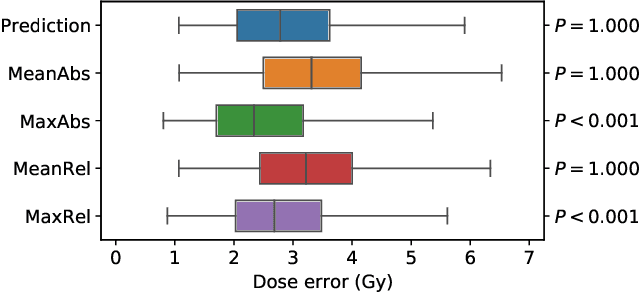
Abstract:We establish an open framework for developing plan optimization models for knowledge-based planning (KBP) in radiotherapy. Our framework includes reference plans for 100 patients with head-and-neck cancer and high-quality dose predictions from 19 KBP models that were developed by different research groups during the OpenKBP Grand Challenge. The dose predictions were input to four optimization models to form 76 unique KBP pipelines that generated 7600 plans. The predictions and plans were compared to the reference plans via: dose score, which is the average mean absolute voxel-by-voxel difference in dose a model achieved; the deviation in dose-volume histogram (DVH) criterion; and the frequency of clinical planning criteria satisfaction. We also performed a theoretical investigation to justify our dose mimicking models. The range in rank order correlation of the dose score between predictions and their KBP pipelines was 0.50 to 0.62, which indicates that the quality of the predictions is generally positively correlated with the quality of the plans. Additionally, compared to the input predictions, the KBP-generated plans performed significantly better (P<0.05; one-sided Wilcoxon test) on 18 of 23 DVH criteria. Similarly, each optimization model generated plans that satisfied a higher percentage of criteria than the reference plans. Lastly, our theoretical investigation demonstrated that the dose mimicking models generated plans that are also optimal for a conventional planning model. This was the largest international effort to date for evaluating the combination of KBP prediction and optimization models. In the interest of reproducibility, our data and code is freely available at https://github.com/ababier/open-kbp-opt.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge