Baris Bingol
Self-supervised Learning for Segmentation and Quantification of Dopamine Neurons in $\text{Parkinson's Disease}$
Jan 11, 2023Abstract:$\text{Parkinson's Disease}$ (PD) is the second most common neurodegenerative disease in humans. PD is characterized by the gradual loss of dopaminergic neurons in the Substantia Nigra (a part of the mid-brain). Counting the number of dopaminergic neurons in the Substantia Nigra is one of the most important indexes in evaluating drug efficacy in PD animal models. Currently, analyzing and quantifying dopaminergic neurons is conducted manually by experts through analysis of digital pathology images which is laborious, time-consuming, and highly subjective. As such, a reliable and unbiased automated system is demanded for the quantification of dopaminergic neurons in digital pathology images. We propose an end-to-end deep learning framework for the segmentation and quantification of dopaminergic neurons in PD animal models. To the best of knowledge, this is the first machine learning model that detects the cell body of dopaminergic neurons, counts the number of dopaminergic neurons and provides the phenotypic characteristics of individual dopaminergic neurons as a numerical output. Extensive experiments demonstrate the effectiveness of our model in quantifying neurons with a high precision, which can provide quicker turnaround for drug efficacy studies, better understanding of dopaminergic neuronal health status and unbiased results in PD pre-clinical research.
Multiclass Semantic Segmentation to Identify Anatomical Sub-Regions of Brain and Measure Neuronal Health in Parkinson's Disease
Jan 07, 2023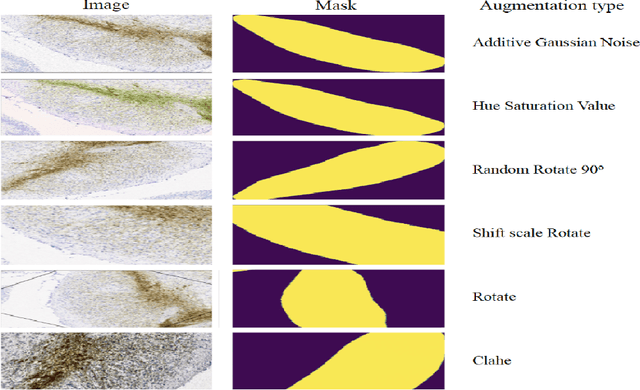

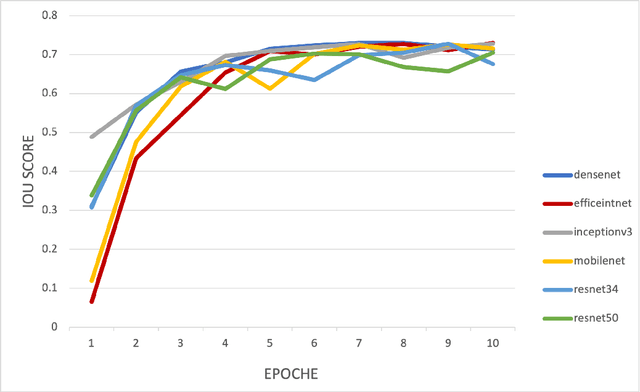

Abstract:Automated segmentation of anatomical sub-regions with high precision has become a necessity to enable the quantification and characterization of cells/ tissues in histology images. Currently, a machine learning model to analyze sub-anatomical regions of the brain to analyze 2D histological images is not available. The scientists rely on manually segmenting anatomical sub-regions of the brain which is extremely time-consuming and prone to labeler-dependent bias. One of the major challenges in accomplishing such a task is the lack of high-quality annotated images that can be used to train a generic artificial intelligence model. In this study, we employed a UNet-based architecture, compared model performance with various combinations of encoders, image sizes, and sample selection techniques. Additionally, to increase the sample set we resorted to data augmentation which provided data diversity and robust learning. In this study, we trained our best fit model on approximately one thousand annotated 2D brain images stained with Nissl/ Haematoxylin and Tyrosine Hydroxylase enzyme (TH, indicator of dopaminergic neuron viability). The dataset comprises of different animal studies enabling the model to be trained on different datasets. The model effectively is able to detect two sub-regions compacta (SNCD) and reticulata (SNr) in all the images. In spite of limited training data, our best model achieves a mean intersection over union (IOU) of 79% and a mean dice coefficient of 87%. In conclusion, the UNet-based model with EffiecientNet as an encoder outperforms all other encoders, resulting in a first of its kind robust model for multiclass segmentation of sub-brain regions in 2D images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge