Antoine Malescot
Inverse Problem Based on a Sparse Representation of Contrast-enhanced Ultrasound Data for in vivo Transcranial Imaging
Jan 18, 2024Abstract:Transcranial ultrasound imaging is currently limited by attenuation and aberration induced by the skull. First used in contrast-enhanced ultrasound (CEUS), highly echoic microbubbles allowed for the development of novel imaging modalities such as ultrasound localization microscopy (ULM). Herein, we develop an inverse problem approach to aberration correction (IPAC) that leverages the sparsity of microbubble signals. We propose to use the \textit{a priori} knowledge of the medium based upon microbubble localization and wave propagation to build a forward model to link the measured signals directly to the aberration function. A standard least-squares inversion is then used to retrieve the aberration function. We first validated IPAC on simulated data of a vascular network using plane wave as well as divergent wave emissions. We then evaluated the reproducibility of IPAC \textit{in vivo} in 5 mouse brains. We showed that aberration correction improved the contrast of CEUS images by 4.6 dB. For ULM images, IPAC yielded sharper vessels, reduced vessel duplications, and improved the resolution from 21.1 $\mu$m to 18.3 $\mu$m. Aberration correction also improved hemodynamic quantification for velocity magnitude and flow direction.
Phase Aberration Correction for in vivo Ultrasound Localization Microscopy Using a Spatiotemporal Complex-Valued Neural Network
Sep 23, 2022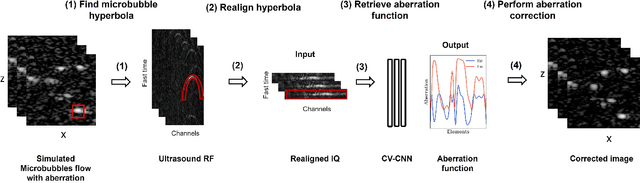
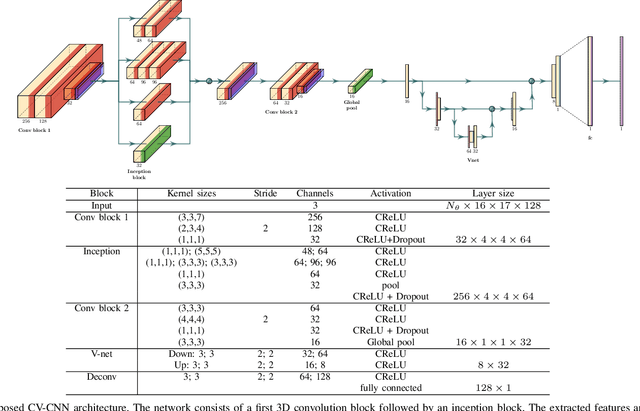
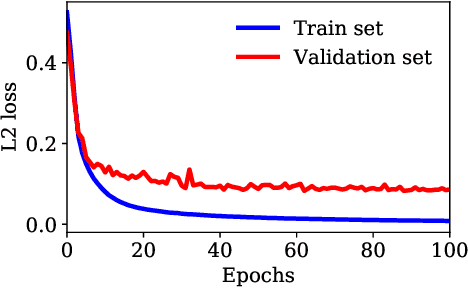
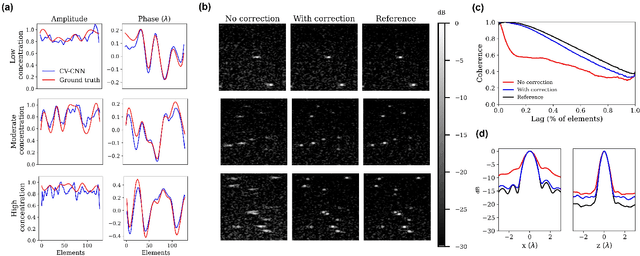
Abstract:Ultrasound Localization Microscopy (ULM) can map microvessels at a resolution of a few micrometers ({\mu}m). Transcranial ULM remains challenging in presence of aberrations caused by the skull, which lead to localization errors. Herein, we propose a deep learning approach based on recently introduced complex-valued convolutional neural networks (CV-CNNs) to retrieve the aberration function, which can then be used to form enhanced images using standard delay-and-sum beamforming. Complex-valued convolutional networks were selected as they can apply time delays through multiplication with in-phase quadrature input data. Predicting the aberration function rather than corrected images also confers enhanced explainability to the network. In addition, 3D spatiotemporal convolutions were used for the network to leverage entire microbubble tracks. For training and validation, we used an anatomically and hemodynamically realistic mouse brain microvascular network model to simulate the flow of microbubbles in presence of aberration. We then confirmed the capability of our network to generalize to transcranial in vivo data in the mouse brain (n=2). Qualitatively, vascular reconstructions using a pixel-wise predicted aberration function included additional and sharper vessels. The spatial resolution was evaluated by using the Fourier ring correlation (FRC). After correction, we measured a resolution of 16.7 {\mu}m in vivo, representing an improvement of up to 27.5 %. This work leads to different applications for complex-valued convolutions in biomedical imaging and strategies to perform transcranial ULM.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge