Anders O. Garlid
on behalf of CURE-CKD
Automated Dynamic Bayesian Networks for Predicting Acute Kidney Injury Before Onset
Apr 20, 2023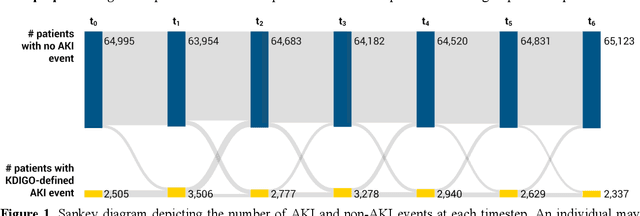



Abstract:Several algorithms for learning the structure of dynamic Bayesian networks (DBNs) require an a priori ordering of variables, which influences the determined graph topology. However, it is often unclear how to determine this order if feature importance is unknown, especially as an exhaustive search is usually impractical. In this paper, we introduce Ranking Approaches for Unknown Structures (RAUS), an automated framework to systematically inform variable ordering and learn networks end-to-end. RAUS leverages existing statistical methods (Cramers V, chi-squared test, and information gain) to compare variable ordering, resultant generated network topologies, and DBN performance. RAUS enables end-users with limited DBN expertise to implement models via command line interface. We evaluate RAUS on the task of predicting impending acute kidney injury (AKI) from inpatient clinical laboratory data. Longitudinal observations from 67,460 patients were collected from our electronic health record (EHR) and Kidney Disease Improving Global Outcomes (KDIGO) criteria were then applied to define AKI events. RAUS learns multiple DBNs simultaneously to predict a future AKI event at different time points (i.e., 24-, 48-, 72-hours in advance of AKI). We also compared the results of the learned AKI prediction models and variable orderings to baseline techniques (logistic regression, random forests, and extreme gradient boosting). The DBNs generated by RAUS achieved 73-83% area under the receiver operating characteristic curve (AUCROC) within 24-hours before AKI; and 71-79% AUCROC within 48-hours before AKI of any stage in a 7-day observation window. Insights from this automated framework can help efficiently implement and interpret DBNs for clinical decision support. The source code for RAUS is available in GitHub at https://github.com/dgrdn08/RAUS .
Design considerations for a hierarchical semantic compositional framework for medical natural language understanding
Apr 05, 2022



Abstract:Medical natural language processing (NLP) systems are a key enabling technology for transforming Big Data from clinical report repositories to information used to support disease models and validate intervention methods. However, current medical NLP systems fall considerably short when faced with the task of logically interpreting clinical text. In this paper, we describe a framework inspired by mechanisms of human cognition in an attempt to jump the NLP performance curve. The design centers about a hierarchical semantic compositional model (HSCM) which provides an internal substrate for guiding the interpretation process. The paper describes insights from four key cognitive aspects including semantic memory, semantic composition, semantic activation, and hierarchical predictive coding. We discuss the design of a generative semantic model and an associated semantic parser used to transform a free-text sentence into a logical representation of its meaning. The paper discusses supportive and antagonistic arguments for the key features of the architecture as a long-term foundational framework.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge