Amir Mohammad Naderi
Towards Precision Cardiovascular Analysis in Zebrafish: The ZACAF Paradigm
Feb 15, 2024Abstract:Quantifying cardiovascular parameters like ejection fraction in zebrafish as a host of biological investigations has been extensively studied. Since current manual monitoring techniques are time-consuming and fallible, several image processing frameworks have been proposed to automate the process. Most of these works rely on supervised deep-learning architectures. However, supervised methods tend to be overfitted on their training dataset. This means that applying the same framework to new data with different imaging setups and mutant types can severely decrease performance. We have developed a Zebrafish Automatic Cardiovascular Assessment Framework (ZACAF) to quantify the cardiac function in zebrafish. In this work, we further applied data augmentation, Transfer Learning (TL), and Test Time Augmentation (TTA) to ZACAF to improve the performance for the quantification of cardiovascular function quantification in zebrafish. This strategy can be integrated with the available frameworks to aid other researchers. We demonstrate that using TL, even with a constrained dataset, the model can be refined to accommodate a novel microscope setup, encompassing diverse mutant types and accommodating various video recording protocols. Additionally, as users engage in successive rounds of TL, the model is anticipated to undergo substantial enhancements in both generalizability and accuracy. Finally, we applied this approach to assess the cardiovascular function in nrap mutant zebrafish, a model of cardiomyopathy.
Deep learning-based framework for cardiac function assessment in embryonic zebrafish from heart beating videos
Feb 24, 2021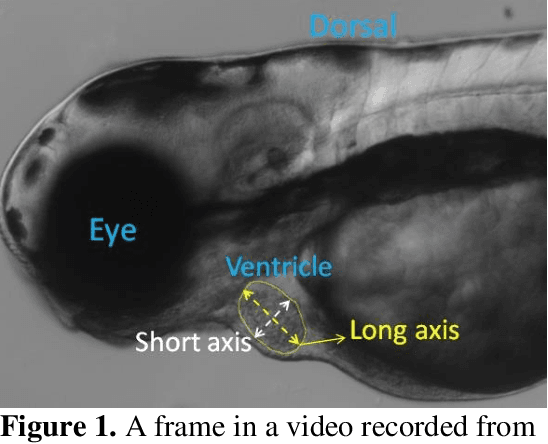
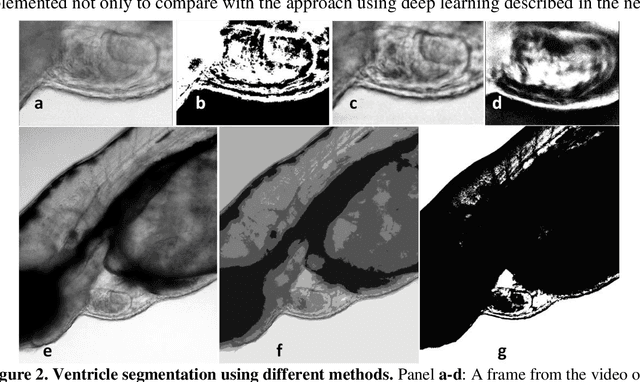
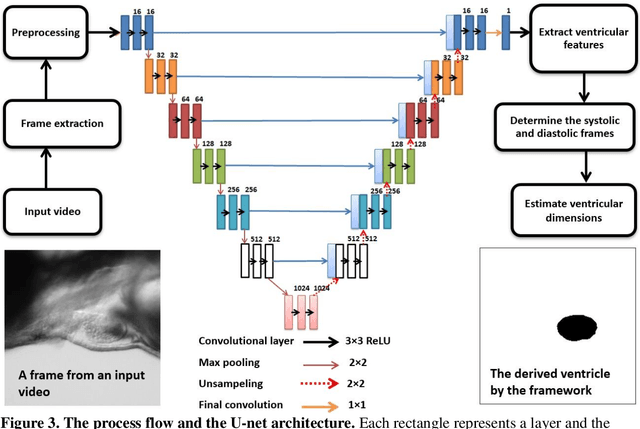
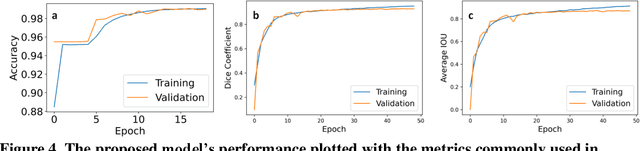
Abstract:Zebrafish is a powerful and widely-used model system for a host of biological investigations including cardiovascular studies and genetic screening. Zebrafish are readily assessable during developmental stages; however, the current methods for quantification and monitoring of cardiac functions mostly involve tedious manual work and inconsistent estimations. In this paper, we developed and validated a Zebrafish Automatic Cardiovascular Assessment Framework (ZACAF) based on a U-net deep learning model for automated assessment of cardiovascular indices, such as ejection fraction (EF) and fractional shortening (FS) from microscopic videos of wildtype and cardiomyopathy mutant zebrafish embryos. Our approach yielded favorable performance with accuracy above 90% compared with manual processing. We used only black and white regular microscopic recordings with frame rates of 5-20 frames per second (fps); thus, the framework could be widely applicable with any laboratory resources and infrastructure. Most importantly, the automatic feature holds promise to enable efficient, consistent and reliable processing and analysis capacity for large amounts of videos, which can be generated by diverse collaborating teams.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge