Alex J. Noble
Learning to automate cryo-electron microscopy data collection with Ptolemy
Dec 01, 2021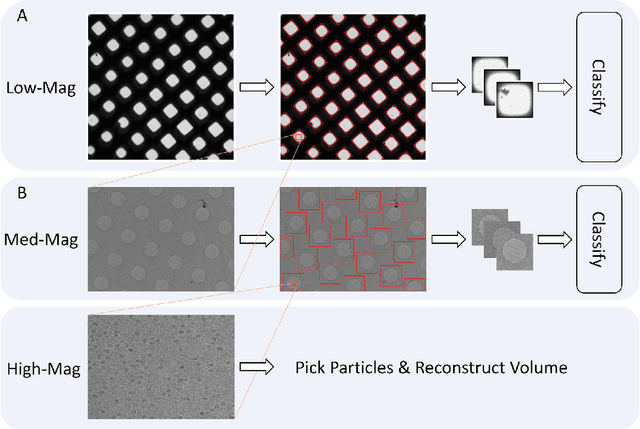
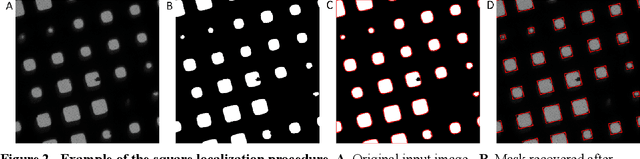
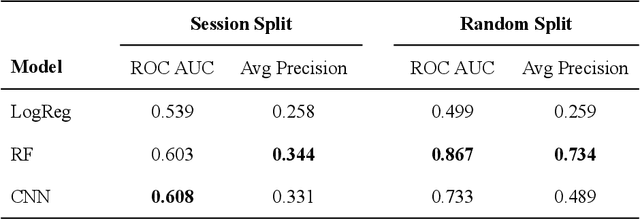
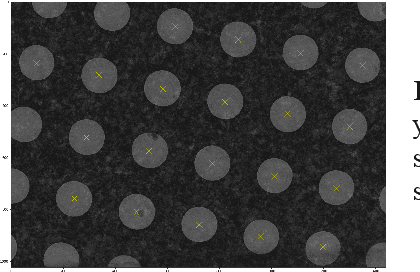
Abstract:Over the past decade, cryogenic electron microscopy (cryo-EM) has emerged as a primary method for determining near-native, near-atomic resolution 3D structures of biological macromolecules. In order to meet increasing demand for cryo-EM, automated methods to improve throughput and efficiency while lowering costs are needed. Currently, the process of collecting high-magnification cryo-EM micrographs, data collection, requires human input and manual tuning of parameters, as expert operators must navigate low- and medium-magnification images to find good high-magnification collection locations. Automating this is non-trivial: the images suffer from low signal-to-noise ratio and are affected by a range of experimental parameters that can differ for each collection session. Here, we use various computer vision algorithms, including mixture models, convolutional neural networks (CNNs), and U-Nets to develop the first pipeline to automate low- and medium-magnification targeting with purpose-built algorithms. Learned models in this pipeline are trained on a large internal dataset of images from real world cryo-EM data collection sessions, labeled with locations that were selected by operators. Using these models, we show that we can effectively detect and classify regions of interest (ROIs) in low- and medium-magnification images, and can generalize to unseen sessions, as well as to images captured using different microscopes from external facilities. We expect our pipeline, Ptolemy, will be both immediately useful as a tool for automation of cryo-EM data collection, and serve as a foundation for future advanced methods for efficient and automated cryo-EM microscopy.
Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs
Oct 08, 2018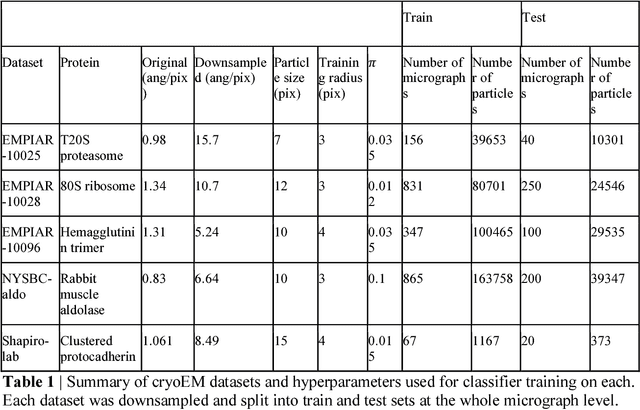
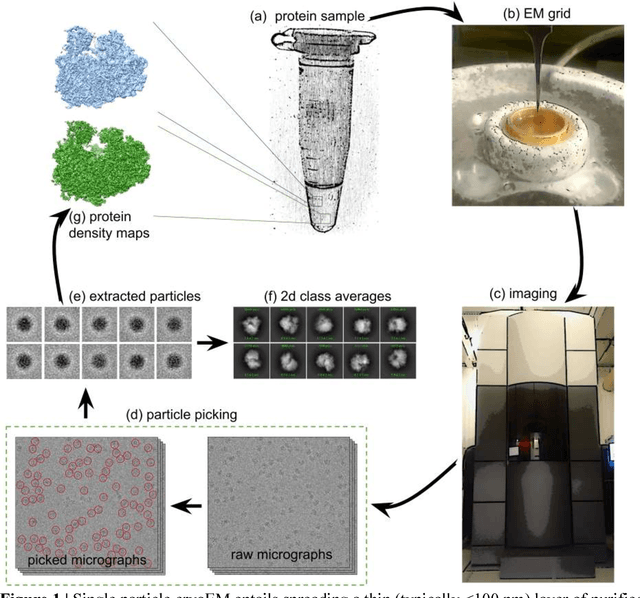
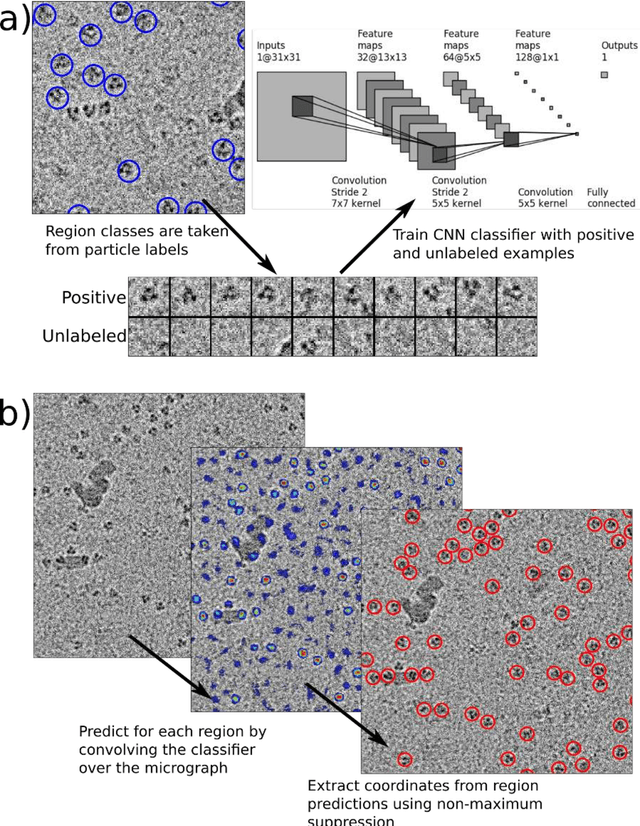
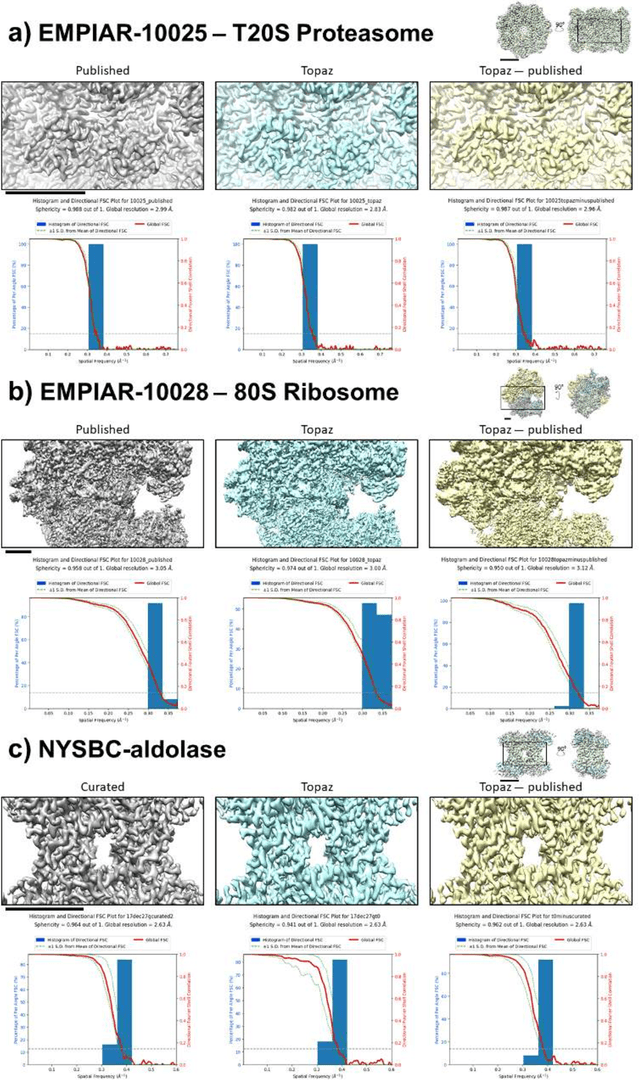
Abstract:Cryo-electron microscopy (cryoEM) is an increasingly popular method for protein structure determination. However, identifying a sufficient number of particles for analysis (often >100,000) can take months of manual effort. Current computational approaches are limited by high false positive rates and require significant ad-hoc post-processing, especially for unusually shaped particles. To address this shortcoming, we develop Topaz, an efficient and accurate particle picking pipeline using neural networks trained with few labeled particles by newly leveraging the remaining unlabeled particles through the framework of positive-unlabeled (PU) learning. Remarkably, despite using minimal labeled particles, Topaz allows us to improve reconstruction resolution by up to 0.15 {\AA} over published particles on three public cryoEM datasets without any post-processing. Furthermore, we show that our novel generalized-expectation criteria approach to PU learning outperforms existing general PU learning approaches when applied to particle detection, especially for challenging datasets of non-globular proteins. We expect Topaz to be an essential component of cryoEM analysis.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge