Learning to automate cryo-electron microscopy data collection with Ptolemy
Paper and Code
Dec 01, 2021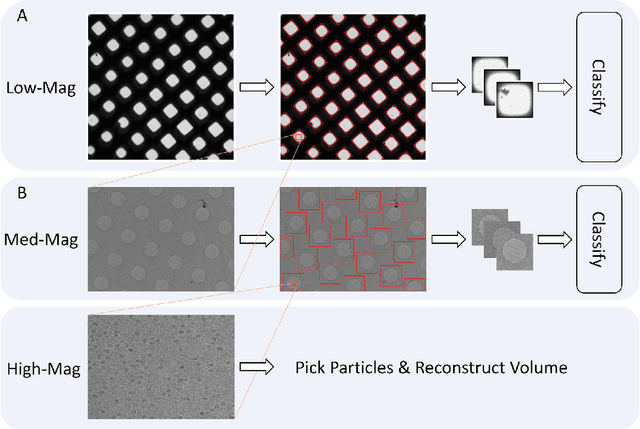
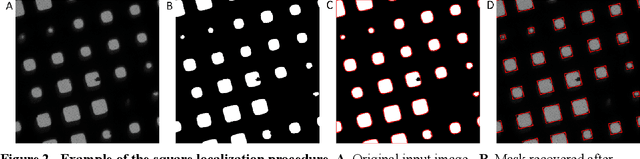
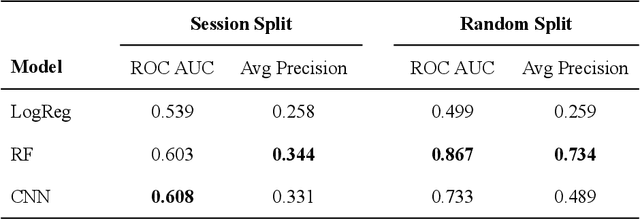
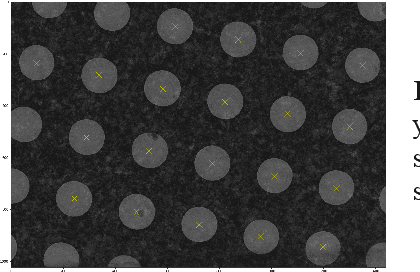
Over the past decade, cryogenic electron microscopy (cryo-EM) has emerged as a primary method for determining near-native, near-atomic resolution 3D structures of biological macromolecules. In order to meet increasing demand for cryo-EM, automated methods to improve throughput and efficiency while lowering costs are needed. Currently, the process of collecting high-magnification cryo-EM micrographs, data collection, requires human input and manual tuning of parameters, as expert operators must navigate low- and medium-magnification images to find good high-magnification collection locations. Automating this is non-trivial: the images suffer from low signal-to-noise ratio and are affected by a range of experimental parameters that can differ for each collection session. Here, we use various computer vision algorithms, including mixture models, convolutional neural networks (CNNs), and U-Nets to develop the first pipeline to automate low- and medium-magnification targeting with purpose-built algorithms. Learned models in this pipeline are trained on a large internal dataset of images from real world cryo-EM data collection sessions, labeled with locations that were selected by operators. Using these models, we show that we can effectively detect and classify regions of interest (ROIs) in low- and medium-magnification images, and can generalize to unseen sessions, as well as to images captured using different microscopes from external facilities. We expect our pipeline, Ptolemy, will be both immediately useful as a tool for automation of cryo-EM data collection, and serve as a foundation for future advanced methods for efficient and automated cryo-EM microscopy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge