Adam Dorfman
Insights into Data through Model Behaviour: An Explainability-driven Strategy for Data Auditing for Responsible Computer Vision Applications
Jun 16, 2021



Abstract:In this study, we take a departure and explore an explainability-driven strategy to data auditing, where actionable insights into the data at hand are discovered through the eyes of quantitative explainability on the behaviour of a dummy model prototype when exposed to data. We demonstrate this strategy by auditing two popular medical benchmark datasets, and discover hidden data quality issues that lead deep learning models to make predictions for the wrong reasons. The actionable insights gained from this explainability driven data auditing strategy is then leveraged to address the discovered issues to enable the creation of high-performing deep learning models with appropriate prediction behaviour. The hope is that such an explainability-driven strategy can be complimentary to data-driven strategies to facilitate for more responsible development of machine learning algorithms for computer vision applications.
Fibrosis-Net: A Tailored Deep Convolutional Neural Network Design for Prediction of Pulmonary Fibrosis Progression from Chest CT Images
Mar 06, 2021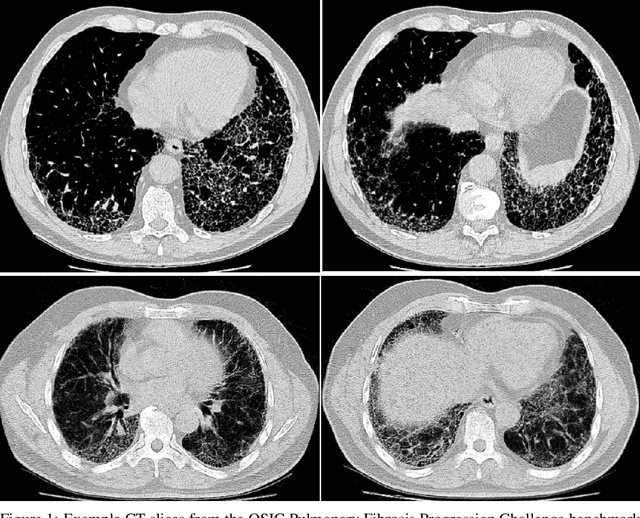
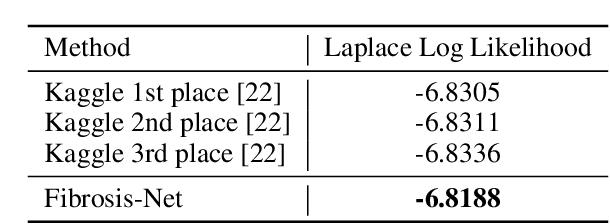
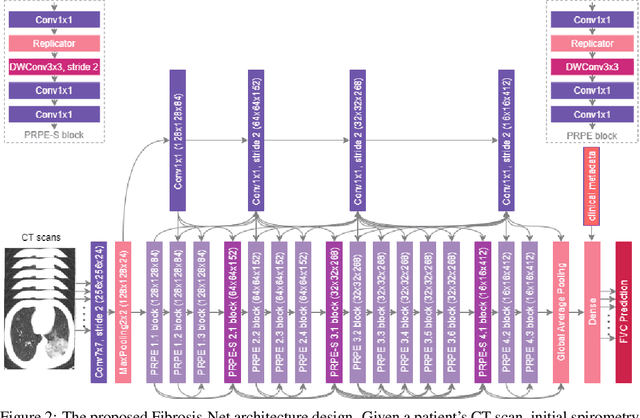
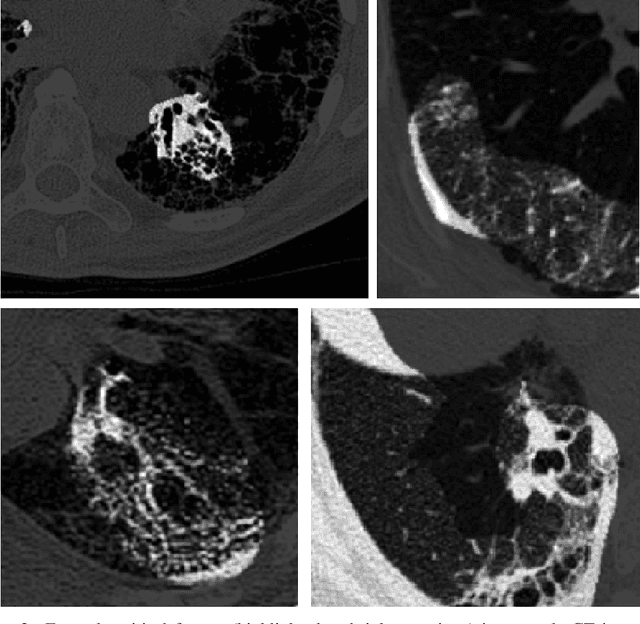
Abstract:Pulmonary fibrosis is a devastating chronic lung disease that causes irreparable lung tissue scarring and damage, resulting in progressive loss in lung capacity and has no known cure. A critical step in the treatment and management of pulmonary fibrosis is the assessment of lung function decline, with computed tomography (CT) imaging being a particularly effective method for determining the extent of lung damage caused by pulmonary fibrosis. Motivated by this, we introduce Fibrosis-Net, a deep convolutional neural network design tailored for the prediction of pulmonary fibrosis progression from chest CT images. More specifically, machine-driven design exploration was leveraged to determine a strong architectural design for CT lung analysis, upon which we build a customized network design tailored for predicting forced vital capacity (FVC) based on a patient's CT scan, initial spirometry measurement, and clinical metadata. Finally, we leverage an explainability-driven performance validation strategy to study the decision-making behaviour of Fibrosis-Net as to verify that predictions are based on relevant visual indicators in CT images. Experiments using the OSIC Pulmonary Fibrosis Progression Challenge benchmark dataset showed that the proposed Fibrosis-Net is able to achieve a significantly higher modified Laplace Log Likelihood score than the winning solutions on the challenge leaderboard. Furthermore, explainability-driven performance validation demonstrated that the proposed Fibrosis-Net exhibits correct decision-making behaviour by leveraging clinically-relevant visual indicators in CT images when making predictions on pulmonary fibrosis progress. While Fibrosis-Net is not yet a production-ready clinical assessment solution, we hope that releasing the model in open source manner will encourage researchers, clinicians, and citizen data scientists alike to leverage and build upon it.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge