Abeshek A
A Novel Adaptive Hybrid Focal-Entropy Loss for Enhancing Diabetic Retinopathy Detection Using Convolutional Neural Networks
Nov 16, 2024



Abstract:Diabetic retinopathy is a leading cause of blindness around the world and demands precise AI-based diagnostic tools. Traditional loss functions in multi-class classification, such as Categorical Cross-Entropy (CCE), are very common but break down with class imbalance, especially in cases with inherently challenging or overlapping classes, which leads to biased and less sensitive models. Since a heavy imbalance exists in the number of examples for higher severity stage 4 diabetic retinopathy, etc., classes compared to those very early stages like class 0, achieving class balance is key. For this purpose, we propose the Adaptive Hybrid Focal-Entropy Loss which combines the ideas of focal loss and entropy loss with adaptive weighting in order to focus on minority classes and highlight the challenging samples. The state-of-the art models applied for diabetic retinopathy detection with AHFE revealed good performance improvements, indicating the top performances of ResNet50 at 99.79%, DenseNet121 at 98.86%, Xception at 98.92%, MobileNetV2 at 97.84%, and InceptionV3 at 93.62% accuracy. This sheds light into how AHFE promotes enhancement in AI-driven diagnostics for complex and imbalanced medical datasets.
An Integrated Deep Learning Framework for Effective Brain Tumor Localization, Segmentation, and Classification from Magnetic Resonance Images
Sep 25, 2024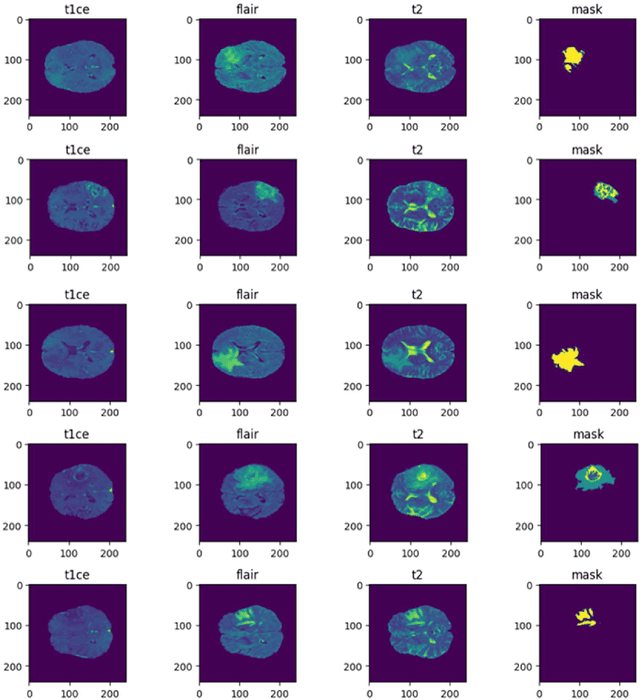


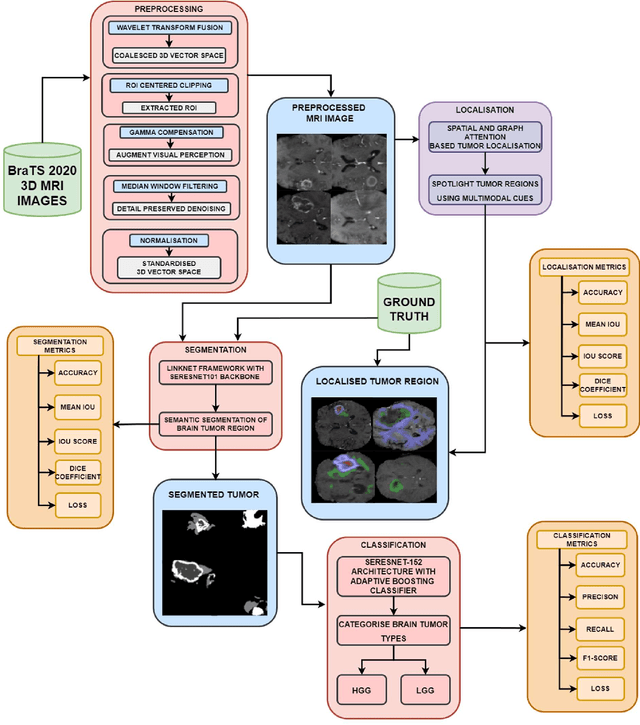
Abstract:Tumors in the brain result from abnormal cell growth within the brain tissue, arising from various types of brain cells. When left undiagnosed, they lead to severe neurological deficits such as cognitive impairment, motor dysfunction, and sensory loss. As the tumor grows, it causes an increase in intracranial pressure, potentially leading to life-threatening complications such as brain herniation. Therefore, early detection and treatment are necessary to manage the complications caused by such tumors to slow down their growth. Numerous works involving deep learning (DL) and artificial intelligence (AI) are being carried out to assist physicians in early diagnosis by utilizing the scans obtained through Magnetic Resonance Imaging (MRI). Our research proposes DL frameworks for localizing, segmenting, and classifying the grade of these gliomas from MRI images to solve this critical issue. In our localization framework, we enhance the LinkNet framework with a VGG19- inspired encoder architecture for improved multimodal tumor feature extraction, along with spatial and graph attention mechanisms to refine feature focus and inter-feature relationships. Following this, we integrated the SeResNet101 CNN model as the encoder backbone into the LinkNet framework for tumor segmentation, which achieved an IoU Score of 96%. To classify the segmented tumors, we combined the SeResNet152 feature extractor with an Adaptive Boosting classifier, which yielded an accuracy of 98.53%. Our proposed models demonstrated promising results, with the potential to advance medical AI by enabling early diagnosis and providing more accurate treatment options for patients.
Leveraging SeNet and ResNet Synergy within an Encoder-Decoder Architecture for Glioma Detection
Sep 01, 2024



Abstract:Brain tumors are abnormalities that can severely impact a patient's health, leading to life-threatening conditions such as cancer. These can result in various debilitating effects, including neurological issues, cognitive impairment, motor and sensory deficits, as well as emotional and behavioral changes. These symptoms significantly affect a patient's quality of life, making early diagnosis and timely treatment essential to prevent further deterioration. However, accurately segmenting the tumor region from medical images, particularly MRI scans, is a challenging and time-consuming task that requires the expertise of radiologists. Manual segmentation can also be prone to human errors. To address these challenges, this research leverages the synergy of SeNet and ResNet architectures within an encoder-decoder framework, designed specifically for glioma detection and segmentation. The proposed model incorporates the power of SeResNet-152 as the backbone, integrated into a robust encoder-decoder structure to enhance feature extraction and improve segmentation accuracy. This novel approach significantly reduces the dependency on manual tasks and improves the precision of tumor identification. Evaluation of the model demonstrates strong performance, achieving 87% in Dice Coefficient, 89.12% in accuracy, 88% in IoU score, and 82% in mean IoU score, showcasing its effectiveness in tackling the complex problem of brain tumor segmentation.
A Channel Attention-Driven Hybrid CNN Framework for Paddy Leaf Disease Detection
Jul 16, 2024



Abstract:Farmers face various challenges when it comes to identifying diseases in rice leaves during their early stages of growth, which is a major reason for poor produce. Therefore, early and accurate disease identification is important in agriculture to avoid crop loss and improve cultivation. In this research, we propose a novel hybrid deep learning (DL) classifier designed by extending the Squeeze-and-Excitation network architecture with a channel attention mechanism and the Swish ReLU activation function. The channel attention mechanism in our proposed model identifies the most important feature channels required for classification during feature extraction and selection. The dying ReLU problem is mitigated by utilizing the Swish ReLU activation function, and the Squeeze-andExcitation blocks improve information propagation and cross-channel interaction. Upon evaluation, our model achieved a high F1-score of 99.76% and an accuracy of 99.74%, surpassing the performance of existing models. These outcomes demonstrate the potential of state-of-the-art DL techniques in agriculture, contributing to the advancement of more efficient and reliable disease detection systems.
A Dual-Attention Aware Deep Convolutional Neural Network for Early Alzheimer's Detection
Jul 15, 2024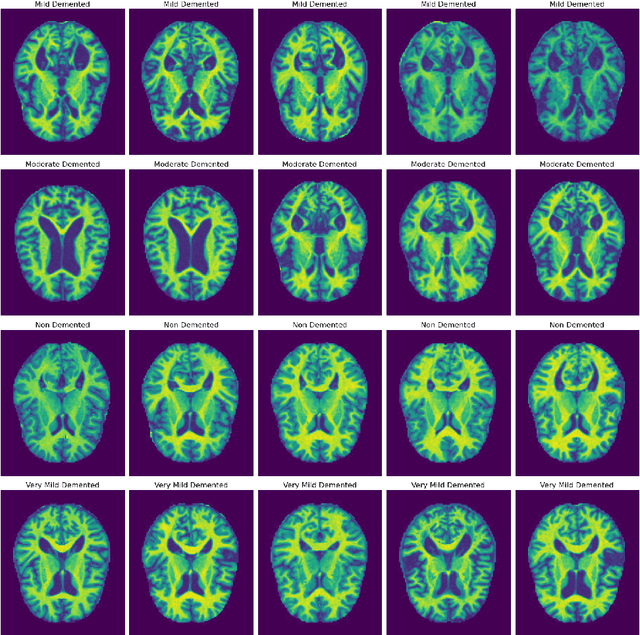



Abstract:Alzheimer's disease (AD) represents the primary form of neurodegeneration, impacting millions of individuals each year and causing progressive cognitive decline. Accurately diagnosing and classifying AD using neuroimaging data presents ongoing challenges in medicine, necessitating advanced interventions that will enhance treatment measures. In this research, we introduce a dual attention enhanced deep learning (DL) framework for classifying AD from neuroimaging data. Combined spatial and self-attention mechanisms play a vital role in emphasizing focus on neurofibrillary tangles and amyloid plaques from the MRI images, which are difficult to discern with regular imaging techniques. Results demonstrate that our model yielded remarkable performance in comparison to existing state of the art (SOTA) convolutional neural networks (CNNs), with an accuracy of 99.1%. Moreover, it recorded remarkable metrics, with an F1-Score of 99.31%, a precision of 99.24%, and a recall of 99.5%. These results highlight the promise of cutting edge DL methods in medical diagnostics, contributing to highly reliable and more efficient healthcare solutions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge