Abdelrahman Abdelkader
Accessible, At-Home Detection of Parkinson's Disease via Multi-task Video Analysis
Jun 21, 2024Abstract:Limited access to neurological care leads to missed diagnoses of Parkinson's disease (PD), leaving many individuals unidentified and untreated. We trained a novel neural network-based fusion architecture to detect Parkinson's disease (PD) by analyzing features extracted from webcam recordings of three tasks: finger tapping, facial expression (smiling), and speech (uttering a sentence containing all letters of the alphabet). Additionally, the model incorporated Monte Carlo Dropout to improve prediction accuracy by considering uncertainties. The study participants (n = 845, 272 with PD) were randomly split into three sets: 60% for training, 20% for model selection (hyper-parameter tuning), and 20% for final performance evaluation. The dataset consists of 1102 sessions, each session containing videos of all three tasks. Our proposed model achieved significantly better accuracy, area under the ROC curve (AUROC), and sensitivity at non-inferior specificity compared to any single-task model. Withholding uncertain predictions further boosted the performance, achieving 88.0% (95% CI: 87.7% - 88.4%) accuracy, 93.0% (92.8% - 93.2%) AUROC, 79.3% (78.4% - 80.2%) sensitivity, and 92.6% (92.3% - 92.8%) specificity, at the expense of not being able to predict for 2.3% (2.0% - 2.6%) data. Further analysis suggests that the trained model does not exhibit any detectable bias across sex and ethnic subgroups and is most effective for individuals aged between 50 and 80. This accessible, low-cost approach requiring only an internet-enabled device with a webcam and microphone paves the way for convenient PD screening at home, particularly in regions with limited access to clinical specialists.
PARK: Parkinson's Analysis with Remote Kinetic-tasks
Nov 21, 2023

Abstract:We present a web-based framework to screen for Parkinson's disease (PD) by allowing users to perform neurological tests in their homes. Our web framework guides the users to complete three tasks involving speech, facial expression, and finger movements. The task videos are analyzed to classify whether the users show signs of PD. We present the results in an easy-to-understand manner, along with personalized resources to further access to treatment and care. Our framework is accessible by any major web browser, improving global access to neurological care.
Using AI to Measure Parkinson's Disease Severity at Home
Apr 13, 2023Abstract:We present an artificial intelligence system to remotely assess the motor performance of individuals with Parkinson's disease (PD). Participants performed a motor task (i.e., tapping fingers) in front of a webcam, and data from 250 global participants were rated by three expert neurologists following the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS). The neurologists' ratings were highly reliable, with an intra-class correlation coefficient (ICC) of 0.88. We developed computer algorithms to obtain objective measurements that align with the MDS-UPDRS guideline and are strongly correlated with the neurologists' ratings. Our machine learning model trained on these measures outperformed an MDS-UPDRS certified rater, with a mean absolute error (MAE) of 0.59 compared to the rater's MAE of 0.79. However, the model performed slightly worse than the expert neurologists (0.53 MAE). The methodology can be replicated for similar motor tasks, providing the possibility of evaluating individuals with PD and other movement disorders remotely, objectively, and in areas with limited access to neurological care.
Auto-Gait: Automatic Ataxia Risk Assessment with Computer Vision on Gait Task Videos
Mar 15, 2022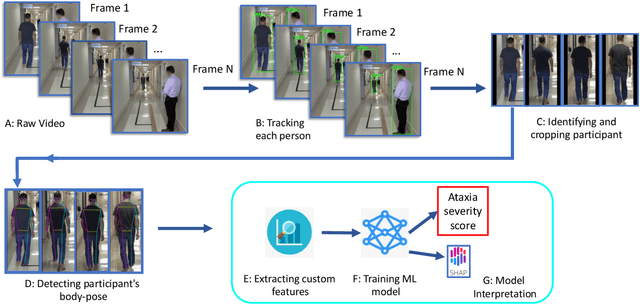

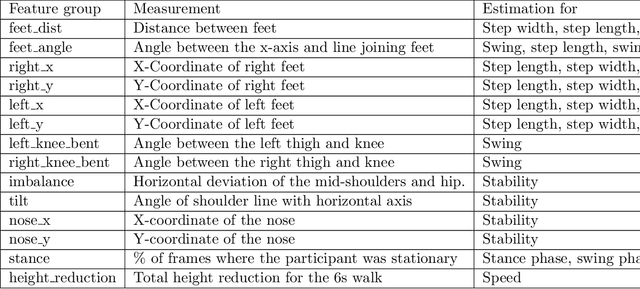
Abstract:In this paper, we investigated whether we can 1) detect participants with ataxia-specific gait characteristics (risk-prediction), and 2) assess severity of ataxia from gait (severity-assessment). We collected 155 videos from 89 participants, 24 controls and 65 diagnosed with (or are pre-manifest) spinocerebellar ataxias (SCAs), performing the gait task of the Scale for the Assessment and Rating of Ataxia (SARA) from 11 medical sites located in 8 different states in the United States. We developed a method to separate the participants from their surroundings and constructed several features to capture gait characteristics like step width, step length, swing, stability, speed, etc. Our risk-prediction model achieves 83.06% accuracy and an 80.23% F1 score. Similarly, our severity-assessment model achieves a mean absolute error (MAE) score of 0.6225 and a Pearson's correlation coefficient score of 0.7268. Our models still performed competitively when evaluated on data from sites not used during training. Furthermore, through feature importance analysis, we found that our models associate wider steps, decreased walking speed, and increased instability with greater ataxia severity, which is consistent with previously established clinical knowledge. Our models create possibilities for remote ataxia assessment in non-clinical settings in the future, which could significantly improve accessibility of ataxia care. Furthermore, our underlying dataset was assembled from a geographically diverse cohort, highlighting its potential to further increase equity. The code used in this study is open to the public, and the anonymized body pose landmark dataset could be released upon approval from our Institutional Review Board (IRB).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge