Aarthi Narayanan
Assessing Concordance between RNA-Seq and NanoString Technologies in Ebola-Infected Nonhuman Primates Using Machine Learning
Oct 30, 2024Abstract:This study evaluates the concordance between RNA sequencing (RNA-Seq) and NanoString technologies for gene expression analysis in non-human primates (NHPs) infected with Ebola virus (EBOV). We performed a detailed comparison of both platforms, demonstrating a strong correlation between them, with Spearman coefficients for 56 out of 62 samples ranging from 0.78 to 0.88, with a mean of 0.83 and a median of 0.85. Bland-Altman analysis further confirmed high consistency, with most measurements falling within 95% confidence limits. A machine learning approach, using the Supervised Magnitude-Altitude Scoring (SMAS) method trained on NanoString data, identified OAS1 as a key marker for distinguishing RT-qPCR positive from negative samples. Remarkably, when applied to RNA-Seq data, OAS1 also achieved 100% accuracy in differentiating infected from uninfected samples using logistic regression, demonstrating its robustness across platforms. Further differential expression analysis identified 12 common genes including ISG15, OAS1, IFI44, IFI27, IFIT2, IFIT3, IFI44L, MX1, MX2, OAS2, RSAD2, and OASL which demonstrated the highest levels of statistical significance and biological relevance across both platforms. Gene Ontology (GO) analysis confirmed that these genes are directly involved in key immune and viral infection pathways, reinforcing their importance in EBOV infection. In addition, RNA-Seq uniquely identified genes such as CASP5, USP18, and DDX60, which play key roles in immune regulation and antiviral defense. This finding highlights the broader detection capabilities of RNA-Seq and underscores the complementary strengths of both platforms in providing a comprehensive and accurate assessment of gene expression changes during Ebola virus infection.
Machine Learning-Based Analysis of Ebola Virus' Impact on Gene Expression in Nonhuman Primates
Jan 22, 2024
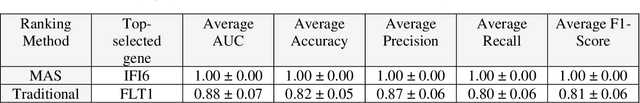

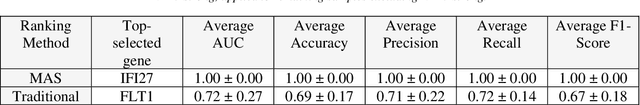
Abstract:This study introduces the Supervised Magnitude-Altitude Scoring (SMAS) methodology, a machine learning-based approach, for analyzing gene expression data obtained from nonhuman primates (NHPs) infected with Ebola virus (EBOV). We utilize a comprehensive dataset of NanoString gene expression profiles from Ebola-infected NHPs, deploying the SMAS system for nuanced host-pathogen interaction analysis. SMAS effectively combines gene selection based on statistical significance and expression changes, employing linear classifiers such as logistic regression to accurately differentiate between RT-qPCR positive and negative NHP samples. A key finding of our research is the identification of IFI6 and IFI27 as critical biomarkers, demonstrating exceptional predictive performance with 100% accuracy and Area Under the Curve (AUC) metrics in classifying various stages of Ebola infection. Alongside IFI6 and IFI27, genes, including MX1, OAS1, and ISG15, were significantly upregulated, highlighting their essential roles in the immune response to EBOV. Our results underscore the efficacy of the SMAS method in revealing complex genetic interactions and response mechanisms during EBOV infection. This research provides valuable insights into EBOV pathogenesis and aids in developing more precise diagnostic tools and therapeutic strategies to address EBOV infection in particular and viral infection in general.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge