Predictive coding in balanced neural networks with noise, chaos and delays
Paper and Code
Jun 25, 2020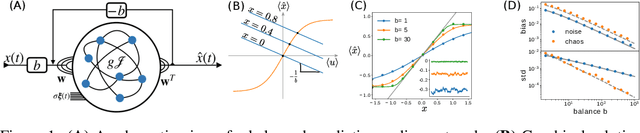
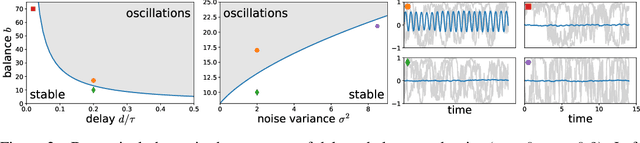
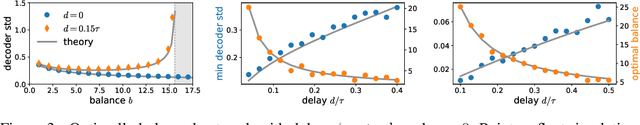
Biological neural networks face a formidable task: performing reliable computations in the face of intrinsic stochasticity in individual neurons, imprecisely specified synaptic connectivity, and nonnegligible delays in synaptic transmission. A common approach to combatting such biological heterogeneity involves averaging over large redundant networks of $N$ neurons resulting in coding errors that decrease classically as $1/\sqrt{N}$. Recent work demonstrated a novel mechanism whereby recurrent spiking networks could efficiently encode dynamic stimuli, achieving a superclassical scaling in which coding errors decrease as $1/N$. This specific mechanism involved two key ideas: predictive coding, and a tight balance, or cancellation between strong feedforward inputs and strong recurrent feedback. However, the theoretical principles governing the efficacy of balanced predictive coding and its robustness to noise, synaptic weight heterogeneity and communication delays remain poorly understood. To discover such principles, we introduce an analytically tractable model of balanced predictive coding, in which the degree of balance and the degree of weight disorder can be dissociated unlike in previous balanced network models, and we develop a mean field theory of coding accuracy. Overall, our work provides and solves a general theoretical framework for dissecting the differential contributions neural noise, synaptic disorder, chaos, synaptic delays, and balance to the fidelity of predictive neural codes, reveals the fundamental role that balance plays in achieving superclassical scaling, and unifies previously disparate models in theoretical neuroscience.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge