Machine learning for classification and quantification of monoclonal antibody preparations for cancer therapy
Paper and Code
May 31, 2017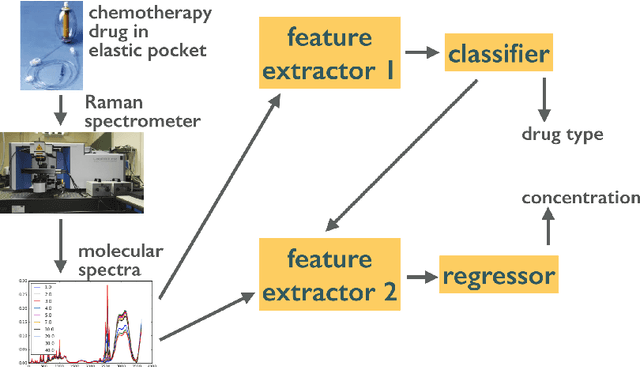
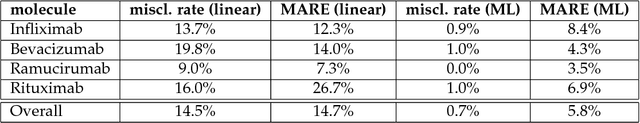
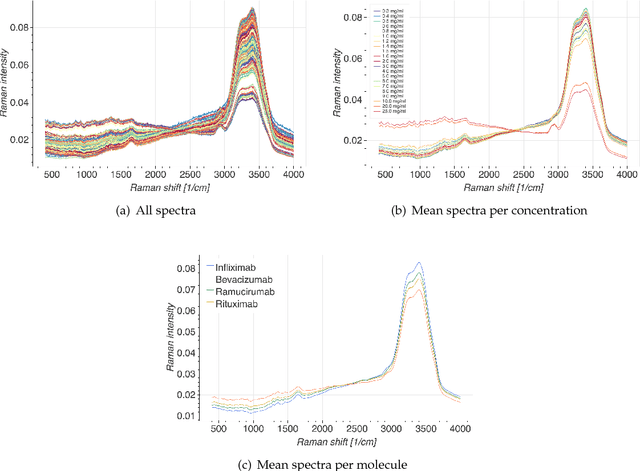
Monoclonal antibodies constitute one of the most important strategies to treat patients suffering from cancers such as hematological malignancies and solid tumors. In order to guarantee the quality of those preparations prepared at hospital, quality control has to be developed. The aim of this study was to explore a noninvasive, nondestructive, and rapid analytical method to ensure the quality of the final preparation without causing any delay in the process. We analyzed four mAbs (Inlfiximab, Bevacizumab, Ramucirumab and Rituximab) diluted at therapeutic concentration in chloride sodium 0.9% using Raman spectroscopy. To reduce the prediction errors obtained with traditional chemometric data analysis, we explored a data-driven approach using statistical machine learning methods where preprocessing and predictive models are jointly optimized. We prepared a data analytics workflow and submitted the problem to a collaborative data challenge platform called Rapid Analytics and Model Prototyping (RAMP). This allowed to use solutions from about 300 data scientists during five days of collaborative work. The prediction of the four mAbs samples was considerably improved with a misclassification rate and the mean error rate of 0.8% and 4%, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge