Hidden yet quantifiable: A lower bound for confounding strength using randomized trials
Paper and Code
Dec 06, 2023
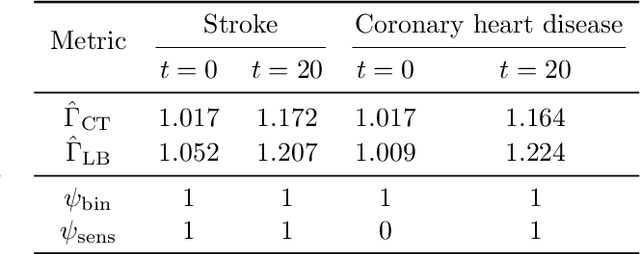
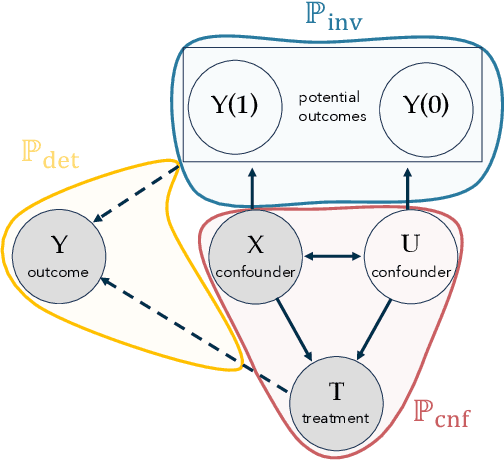
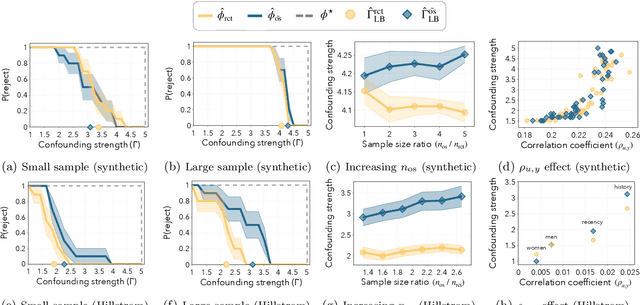
In the era of fast-paced precision medicine, observational studies play a major role in properly evaluating new treatments in clinical practice. Yet, unobserved confounding can significantly compromise causal conclusions drawn from non-randomized data. We propose a novel strategy that leverages randomized trials to quantify unobserved confounding. First, we design a statistical test to detect unobserved confounding with strength above a given threshold. Then, we use the test to estimate an asymptotically valid lower bound on the unobserved confounding strength. We evaluate the power and validity of our statistical test on several synthetic and semi-synthetic datasets. Further, we show how our lower bound can correctly identify the absence and presence of unobserved confounding in a real-world setting.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge