Bayesian optimization assisted unsupervised learning for efficient intra-tumor partitioning in MRI and survival prediction for glioblastoma patients
Paper and Code
Dec 05, 2020

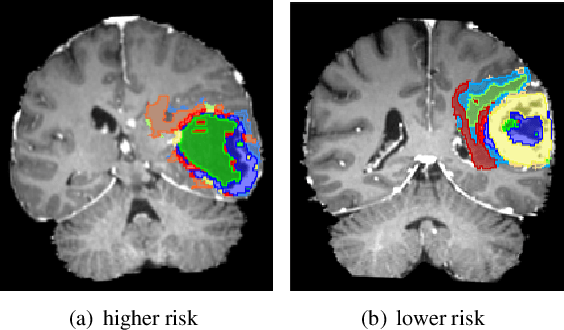
Glioblastoma is profoundly heterogeneous in microstructure and vasculature, which may lead to tumor regional diversity and distinct treatment response. Although successful in tumor sub-region segmentation and survival prediction, radiomics based on machine learning algorithms, is challenged by its robustness, due to the vague intermediate process and track changes. Also, the weak interpretability of the model poses challenges to clinical application. Here we proposed a machine learning framework to semi-automatically fine-tune the clustering algorithms and quantitatively identify stable sub-regions for reliable clinical survival prediction. Hyper-parameters are automatically determined by the global minimum of the trained Gaussian Process (GP) surrogate model through Bayesian optimization(BO) to alleviate the difficulty of tuning parameters for clinical researchers. To enhance the interpretability of the survival prediction model, we incorporated the prior knowledge of intra-tumoral heterogeneity, by segmenting tumor sub-regions and extracting sub-regional features. The results demonstrated that the global minimum of the trained GP surrogate can be used as sub-optimal hyper-parameter solutions for efficient. The sub-regions segmented based on physiological MRI can be applied to predict patient survival, which could enhance the clinical interpretability for the machine learning model.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge