Zvi Friedman
Using Anatomical Markers for Left Ventricular Segmentation of Long Axis Ultrasound Images
Oct 12, 2015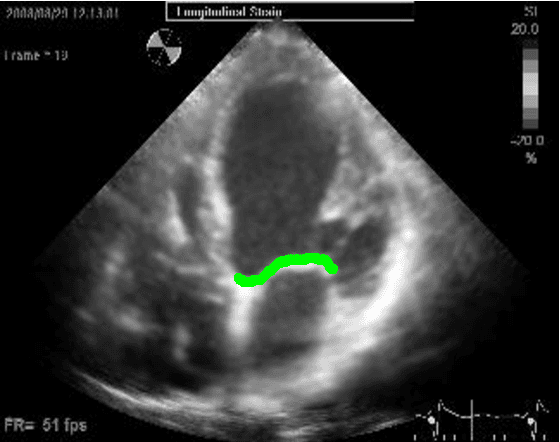
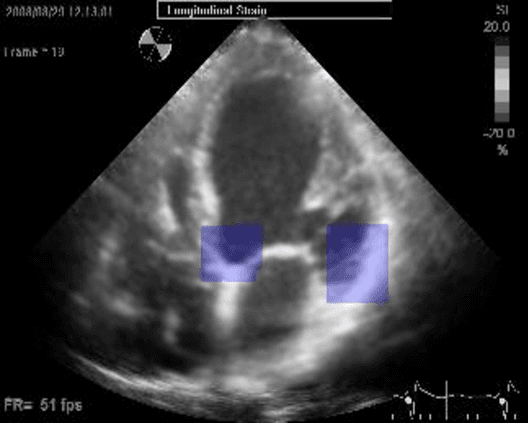
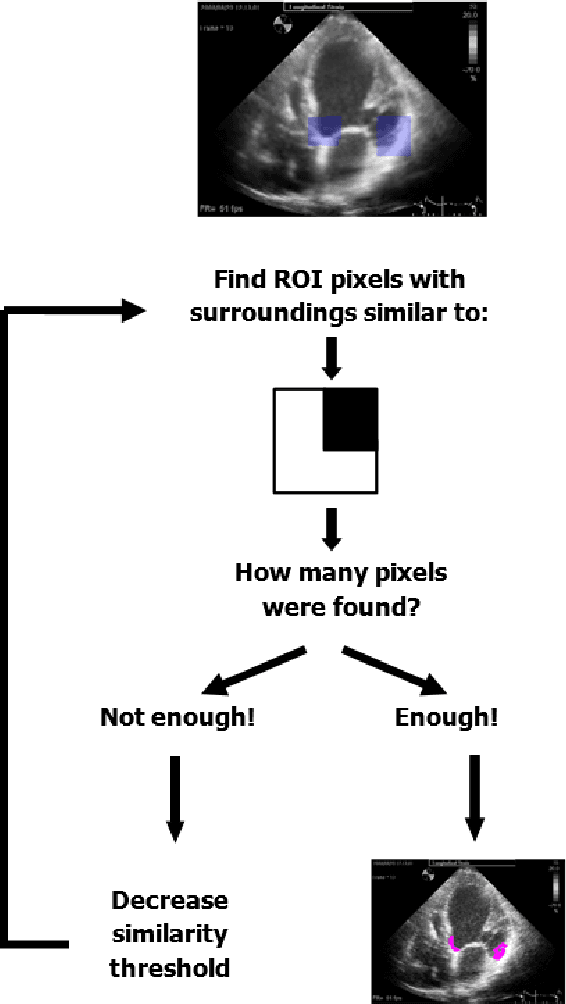
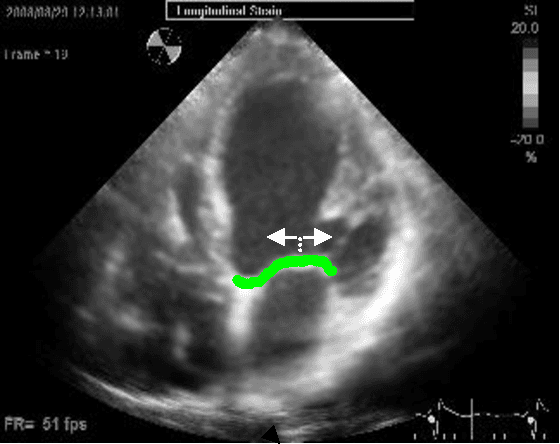
Abstract:Left ventricular segmentation is essential for measuring left ventricular function indices. Segmentation of one or several images requires an initial guess of the contour. It is hypothesized here that creating an initial guess by first detecting anatomical markers, would lead to correct detection of the endocardium. The first step of the algorithm presented here includes automatic detection of the mitral valve. Next, the apex is detected in the same frame. The valve is then tracked throughout the cardiac cycle. Contours passing from the apex to each valve corner are then found using a dynamic programming algorithm. The resulting contour is used as an input to an active contour algorithm. The algorithm was tested on 21 long axis ultrasound clips and showed good agreement with manually traced contours. Thus, this study demonstrates that detection of anatomic markers leads to a reliable initial guess of the left ventricle border.
Compressed Beamforming in Ultrasound Imaging
Apr 10, 2012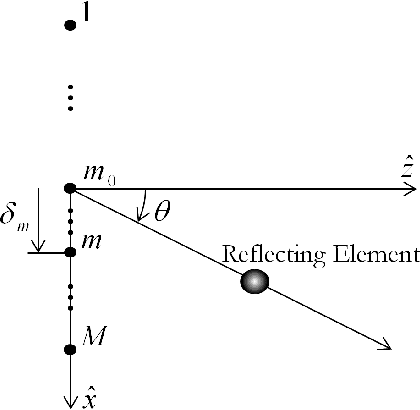
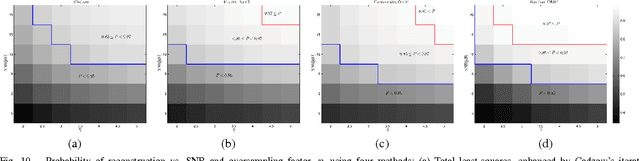
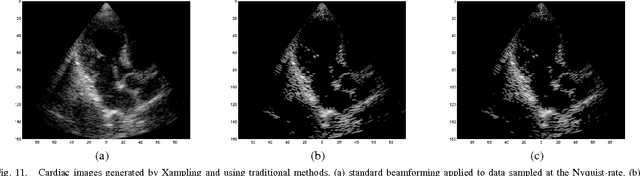
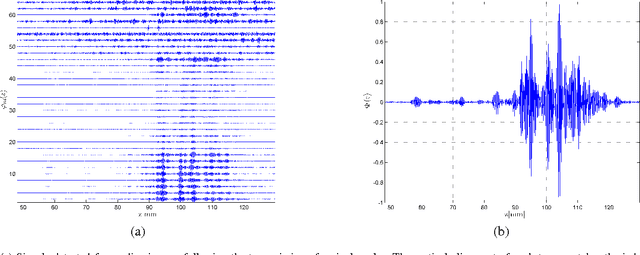
Abstract:Emerging sonography techniques often require increasing the number of transducer elements involved in the imaging process. Consequently, larger amounts of data must be acquired and processed. The significant growth in the amounts of data affects both machinery size and power consumption. Within the classical sampling framework, state of the art systems reduce processing rates by exploiting the bandpass bandwidth of the detected signals. It has been recently shown, that a much more significant sample-rate reduction may be obtained, by treating ultrasound signals within the Finite Rate of Innovation framework. These ideas follow the spirit of Xampling, which combines classic methods from sampling theory with recent developments in Compressed Sensing. Applying such low-rate sampling schemes to individual transducer elements, which detect energy reflected from biological tissues, is limited by the noisy nature of the signals. This often results in erroneous parameter extraction, bringing forward the need to enhance the SNR of the low-rate samples. In our work, we achieve SNR enhancement, by beamforming the sub-Nyquist samples obtained from multiple elements. We refer to this process as "compressed beamforming". Applying it to cardiac ultrasound data, we successfully image macroscopic perturbations, while achieving a nearly eight-fold reduction in sample-rate, compared to standard techniques.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge