Zoltan Patay
Automatic Detection and Segmentation of Postoperative Cerebellar Damage Based on Normalization
Mar 03, 2022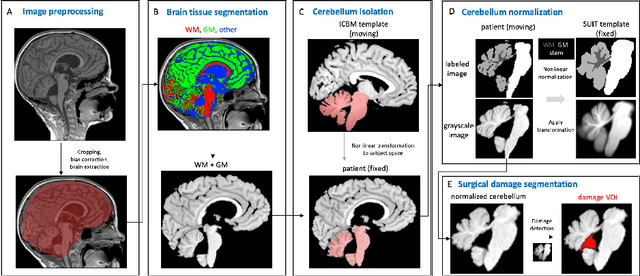
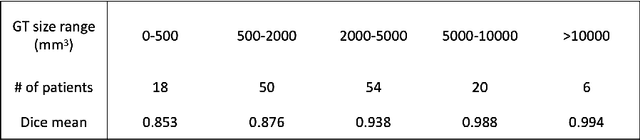
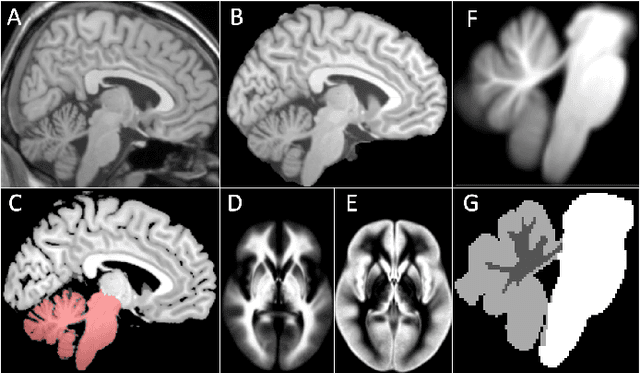

Abstract:Surgical resection is a common procedure in the treatment of pediatric posterior fossa tumors. However, surgical damage is often unavoidable and its association with postoperative complications is not well understood. A reliable localization and measure of cerebellar damage is fundamental to study the relationship between the damaged cerebellar regions and postoperative neurological outcomes. Existing cerebellum normalization methods are not reliable on postoperative scans, therefore current approaches to measure surgical damage rely on manual labelling. In this work, we develop a robust algorithm to automatically detect and measure cerebellum damage due to surgery using postoperative 3D T1 magnetic resonance imaging. In our proposed approach, normal brain tissues are first segmented using a Bayesian algorithm customized for postoperative scans. Next, the cerebellum is isolated by nonlinear registration of a whole brain template to the native space. The isolated cerebellum is then normalized into the spatially unbiased atlas (SUIT) space using anatomical information derived from the previous step. Finally, the damage is detected in the atlas space by comparing the normalized cerebellum and the SUIT template. We evaluated our damage detection tool on postoperative scans of 153 patients diagnosed with medulloblastoma based on inspection by human expects. We also designed a simulation to test the proposed approach without human intervention. Our results show that the proposed approach has superior performance on various scenarios.
A Prior Knowledge Based Tumor and Tumoral Subregion Segmentation Tool for Pediatric Brain Tumors
Sep 30, 2021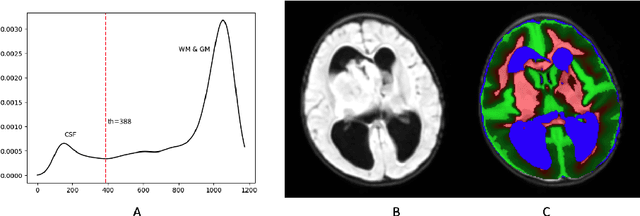
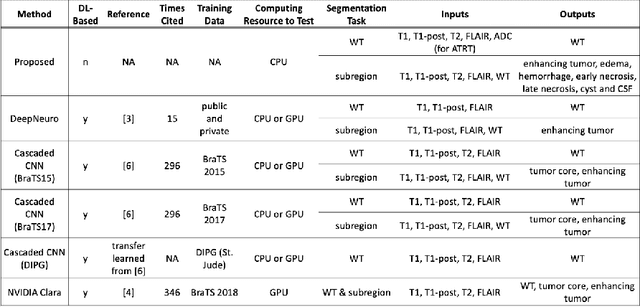
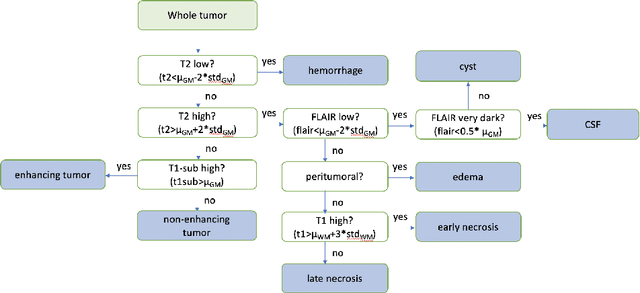
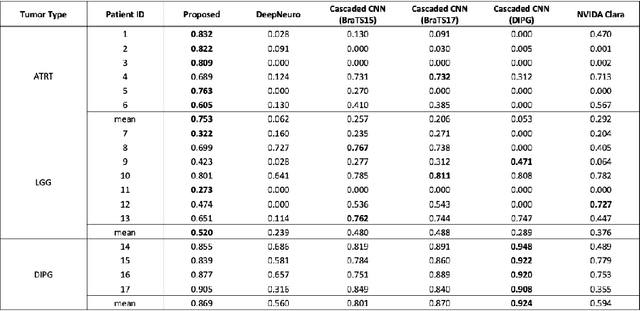
Abstract:In the past few years, deep learning (DL) models have drawn great attention and shown superior performance on brain tumor and subregion segmentation tasks. However, the success is limited to segmentation of adult gliomas, where sufficient data have been collected, manually labeled, and published for training DL models. It is still challenging to segment pediatric tumors, because the appearances are different from adult gliomas. Hence, directly applying a pretained DL model on pediatric data usually generates unacceptable results. Because pediatric data is very limited, both labeled and unlabeled, we present a brain tumor segmentation model that is based on knowledge rather than learning from data. We also provide segmentation of more subregions for super heterogeneous tumor like atypical teratoid rhabdoid tumor (ATRT). Our proposed approach showed superior performance on both whole tumor and subregion segmentation tasks to DL based models on our pediatric data when training data is not available for transfer learning.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge