Tanish Baranwal
Tracking by Predicting 3-D Gaussians Over Time
Dec 30, 2025Abstract:We propose Video Gaussian Masked Autoencoders (Video-GMAE), a self-supervised approach for representation learning that encodes a sequence of images into a set of Gaussian splats moving over time. Representing a video as a set of Gaussians enforces a reasonable inductive bias: that 2-D videos are often consistent projections of a dynamic 3-D scene. We find that tracking emerges when pretraining a network with this architecture. Mapping the trajectory of the learnt Gaussians onto the image plane gives zero-shot tracking performance comparable to state-of-the-art. With small-scale finetuning, our models achieve 34.6% improvement on Kinetics, and 13.1% on Kubric datasets, surpassing existing self-supervised video approaches. The project page and code are publicly available at https://videogmae.org/ and https://github.com/tekotan/video-gmae.
VROOM - Visual Reconstruction over Onboard Multiview
Aug 24, 2025Abstract:We introduce VROOM, a system for reconstructing 3D models of Formula 1 circuits using only onboard camera footage from racecars. Leveraging video data from the 2023 Monaco Grand Prix, we address video challenges such as high-speed motion and sharp cuts in camera frames. Our pipeline analyzes different methods such as DROID-SLAM, AnyCam, and Monst3r and combines preprocessing techniques such as different methods of masking, temporal chunking, and resolution scaling to account for dynamic motion and computational constraints. We show that Vroom is able to partially recover track and vehicle trajectories in complex environments. These findings indicate the feasibility of using onboard video for scalable 4D reconstruction in real-world settings. The project page can be found at https://varun-bharadwaj.github.io/vroom, and our code is available at https://github.com/yajatyadav/vroom.
Fault-Tolerant IoT System Using Software-Based "Digital Twin"
May 29, 2025Abstract:In this article, we present a novel redundancy scheme to realize a fault-tolerant IoT structure for application in high-reliability systems. The proposed fault-tolerant structure uses a centralized data fusion block and triplicated IoT devices, along with software-based "digital twins", that duplicate the function of each of the sensors. In case of a fault in one of the IoT devices, the pertinent digital twin takes over the function of the actual IoT device for some time in the triplicated structure till the faulty device is either replaced or repaired when possible. The use of software-based digital twins as a duplicate for each physical sensor improves the reliability of the operation with minimal increase in the overall system cost.
Machine Learning-Based Anomaly Detection of Correlated Sensor Data: An Integrated Principal Component Analysis-Autoencoder Approach
May 29, 2025Abstract:The growing adoption of IoT systems in industries like transportation, banking, healthcare, and smart energy has increased reliance on sensor networks. However, anomalies in sensor readings can undermine system reliability, making real-time anomaly detection essential. While a large body of research addresses anomaly detection in IoT networks, few studies focus on correlated sensor data streams, such as temperature and pressure within a shared space, especially in resource-constrained environments. To address this, we propose a novel hybrid machine learning approach combining Principal Component Analysis (PCA) and Autoencoders. In this method, PCA continuously monitors sensor data and triggers the Autoencoder when significant variations are detected. This hybrid approach, validated with real-world and simulated data, shows faster response times and fewer false positives. The F1 score of the hybrid method is comparable to Autoencoder, with much faster response time which is driven by PCA.
Discrete vs. Continuous Trade-offs for Generative Models
Dec 26, 2024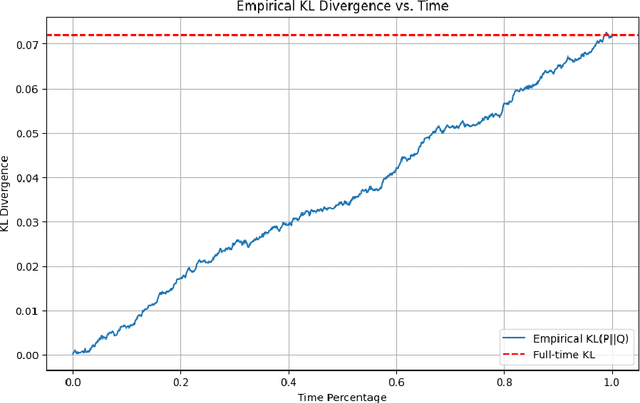
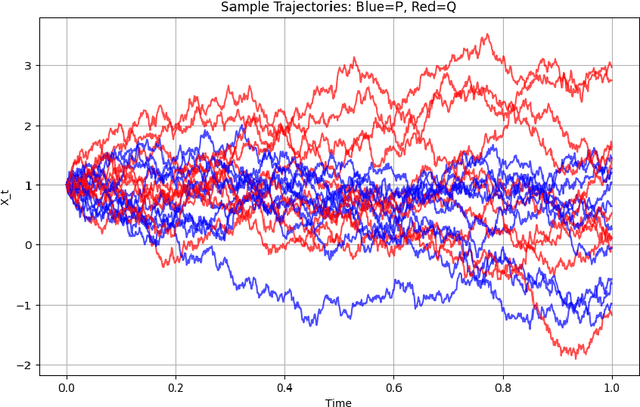
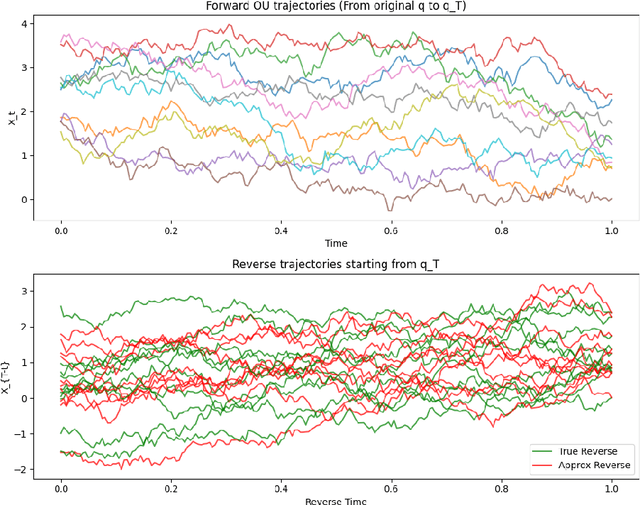
Abstract:This work explores the theoretical and practical foundations of denoising diffusion probabilistic models (DDPMs) and score-based generative models, which leverage stochastic processes and Brownian motion to model complex data distributions. These models employ forward and reverse diffusion processes defined through stochastic differential equations (SDEs) to iteratively add and remove noise, enabling high-quality data generation. By analyzing the performance bounds of these models, we demonstrate how score estimation errors propagate through the reverse process and bound the total variation distance using discrete Girsanov transformations, Pinsker's inequality, and the data processing inequality (DPI) for an information theoretic lens.
Dreaming of Electrical Waves: Generative Modeling of Cardiac Excitation Waves using Diffusion Models
Dec 22, 2023Abstract:Electrical waves in the heart form rotating spiral or scroll waves during life-threatening arrhythmias such as atrial or ventricular fibrillation. The wave dynamics are typically modeled using coupled partial differential equations, which describe reaction-diffusion dynamics in excitable media. More recently, data-driven generative modeling has emerged as an alternative to generate spatio-temporal patterns in physical and biological systems. Here, we explore denoising diffusion probabilistic models for the generative modeling of electrical wave patterns in cardiac tissue. We trained diffusion models with simulated electrical wave patterns to be able to generate such wave patterns in unconditional and conditional generation tasks. For instance, we explored inpainting tasks, such as reconstructing three-dimensional wave dynamics from superficial two-dimensional measurements, and evolving and generating parameter-specific dynamics. We characterized and compared the diffusion-generated solutions to solutions obtained with biophysical models and found that diffusion models learn to replicate spiral and scroll waves dynamics so well that they could serve as an alternative data-driven approach for the modeling of excitation waves in cardiac tissue. For instance, we found that it is possible to initiate ventricular fibrillation (VF) dynamics instantaneously without having to apply pacing protocols in order to induce wavebreak. The VF dynamics can be created in arbitrary ventricular geometries and can be evolved over time. However, we also found that diffusion models `hallucinate' wave patterns when given insufficient constraints. Regardless of these limitations, diffusion models are an interesting and powerful tool with many potential applications in cardiac arrhythmia research and diagnostics.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge