Jan Christoph
Dreaming of Electrical Waves: Generative Modeling of Cardiac Excitation Waves using Diffusion Models
Dec 22, 2023Abstract:Electrical waves in the heart form rotating spiral or scroll waves during life-threatening arrhythmias such as atrial or ventricular fibrillation. The wave dynamics are typically modeled using coupled partial differential equations, which describe reaction-diffusion dynamics in excitable media. More recently, data-driven generative modeling has emerged as an alternative to generate spatio-temporal patterns in physical and biological systems. Here, we explore denoising diffusion probabilistic models for the generative modeling of electrical wave patterns in cardiac tissue. We trained diffusion models with simulated electrical wave patterns to be able to generate such wave patterns in unconditional and conditional generation tasks. For instance, we explored inpainting tasks, such as reconstructing three-dimensional wave dynamics from superficial two-dimensional measurements, and evolving and generating parameter-specific dynamics. We characterized and compared the diffusion-generated solutions to solutions obtained with biophysical models and found that diffusion models learn to replicate spiral and scroll waves dynamics so well that they could serve as an alternative data-driven approach for the modeling of excitation waves in cardiac tissue. For instance, we found that it is possible to initiate ventricular fibrillation (VF) dynamics instantaneously without having to apply pacing protocols in order to induce wavebreak. The VF dynamics can be created in arbitrary ventricular geometries and can be evolved over time. However, we also found that diffusion models `hallucinate' wave patterns when given insufficient constraints. Regardless of these limitations, diffusion models are an interesting and powerful tool with many potential applications in cardiac arrhythmia research and diagnostics.
Panoramic Voltage-Sensitive Optical Mapping of Contracting Hearts using Cooperative Multi-View Motion Tracking with 12 to 24 Cameras
Jul 16, 2023



Abstract:Action potential waves triggering the heart's contractions can be imaged at high spatial and temporal resolutions across the heart surface using voltage-sensitive optical mapping. However, for over three decades, optical mapping has been performed with contraction-inhibited hearts. While it was recently demonstrated that action potential waves can be imaged on parts of the three-dimensional deforming ventricular surface using multi-camera optical mapping, panoramic measurements of action potential waves across the entire beating heart surface remained elusive. Here, we introduce a high-resolution multi-camera optical mapping system consisting of up to 24 high-speed, low-cost cameras with which it is possible to image action potential waves at high resolutions on the entire, strongly deforming ventricular surface of the heart. We imaged isolated hearts inside a custom-designed soccerball-shaped imaging chamber, which facilitates imaging and even illumination with excitation light from all sides in a panoramic fashion. We found that it is possible to image the entire ventricular surface using 12 cameras with 0.5-1.0 megapixels combined resolution. The 12 calibrated cameras generate 1.5 gigabytes of video data per second at imaging speeds of 500 fps, which we process using various computer vision techniques, including three-dimensional cooperative multi-view motion tracking, to generate three-dimensional dynamic reconstructions of the deforming heart surface with corresponding high-resolution voltage-sensitive optical measurements. With our setup, we measured action potential waves at unprecedented resolutions on the contracting three-dimensional surface of rabbit hearts during sinus rhythm, paced rhythm as well as ventricular fibrillation. Our imaging setup defines a new state-of-the-art in the field and can be used to study the heart's electromechanical dynamics during health and disease.
Deep Learning-based Prediction of Electrical Arrhythmia Circuits from Cardiac Motion: An In-Silico Study
May 13, 2023



Abstract:The heart's contraction is caused by electrical excitation which propagates through the heart muscle. It was recently shown that the electrical excitation can be computed from the contractile motion of a simulated piece of heart muscle tissue using deep learning. In cardiac electrophysiology, a primary diagnostic goal is to identify electrical triggers or drivers of heart rhythm disorders. However, using electrical mapping techniques, it is currently impossible to map the three-dimensional morphology of the electrical waves throughout the entire heart muscle, especially during ventricular arrhythmias. Therefore, the approach to calculate or predict electrical excitation from the hearts motion could be a promising alternative diagnostic approach. Here, we demonstrate in computer simulations that it is possible to predict three-dimensional electrical wave dynamics from ventricular deformation mechanics using deep learning. We performed thousands of simulations of electromechanical activation dynamics in ventricular geometries and used the data to train a neural network which subsequently predicts the three-dimensional electrical wave pattern that caused the deformation. We demonstrate that, next to focal wave patterns, even complicated three-dimensional electrical wave patterns can be reconstructed, even if the network has never seen the particular arrhythmia. We show that the deep learning model has the ability to generalize by training it on data generated with the smoothed particle hydrodynamics (SPH) method and subsequently applying it to data generated with the finite element method (FEM). Predictions can be performed in the presence of scars and with significant heterogeneity. Our results suggest that, deep neural networks could be used to calculate intramural action potential wave patterns from imaging data of the motion of the heart muscle.
Reconstruction of Three-dimensional Scroll Wave Chaos in Opaque and Transparent Excitable Media using Deep Neural Networks
Sep 09, 2022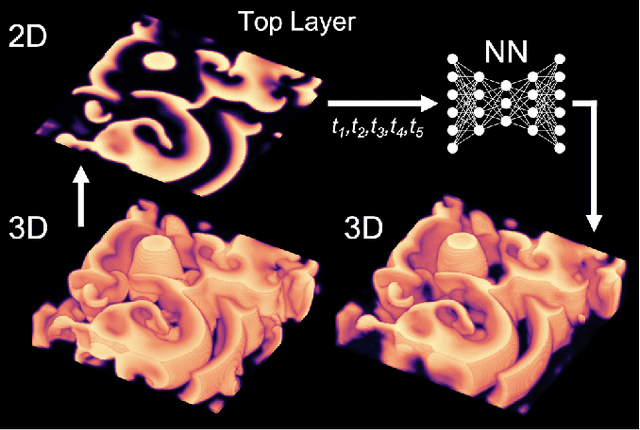
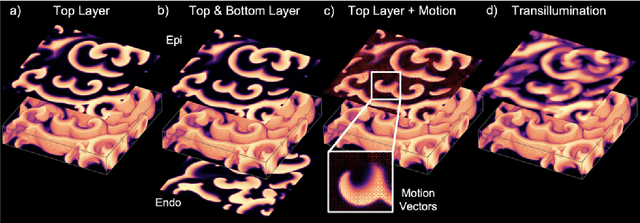


Abstract:Scroll wave chaos is thought to underlie life-threatening ventricular fibrillation. However, currently there is no direct way to measure action potential wave patterns transmurally throughout the thick ventricular heart muscle. Consequently, direct observation of three-dimensional electrical scroll wave chaos remains elusive. Here, we study whether it is possible to reconstruct simulated three-dimensional scroll wave chaos inside a bulk-shaped excitable medium from two-dimensional observations of the wave dynamics on the bulk's surface using deep learning. We trained encoding-decoding convolutional neural networks to predict three-dimensional scroll wave chaos inside opaque and transparent as well as isotropic and anisotropic excitable media from two-dimensional projections or observations of the wave dynamics on the surface. We tested whether observations from one or two opposing surfaces would be sufficient, whether incorporating measurements of the surface deformation improves the reconstruction, and tested the feasibility of predicting the bulk's thickness. We demonstrate that it is possible to fully reconstruct three-dimensional scroll wave chaos in transparent excitable media with anisotropy and to obtain partial reconstructions in opaque excitable media when analyzing two opposing layers of the bulk. We found that anisotropy provides crucial information for neural networks to decode depth, which facilitates the reconstructions. In the future, deep neural networks could be used to visualize transmural action potential wave patterns during ventricular fibrillation from epi- or endocardial recordings.
Rotor Localization and Phase Mapping of Cardiac Excitation Waves using Deep Neural Networks
Sep 22, 2021

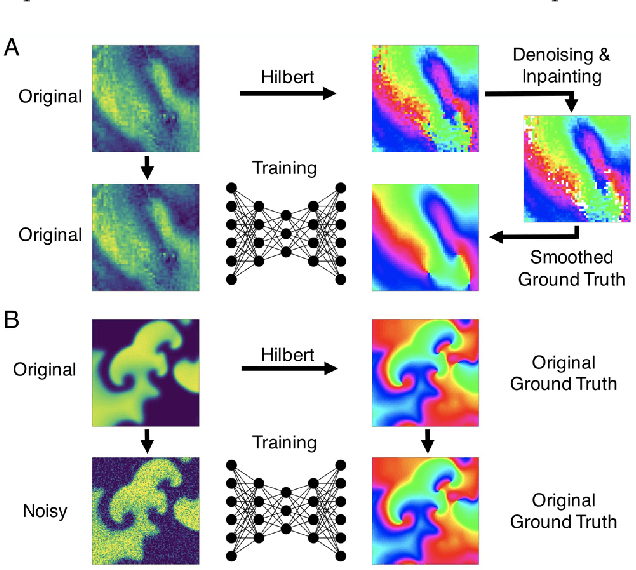
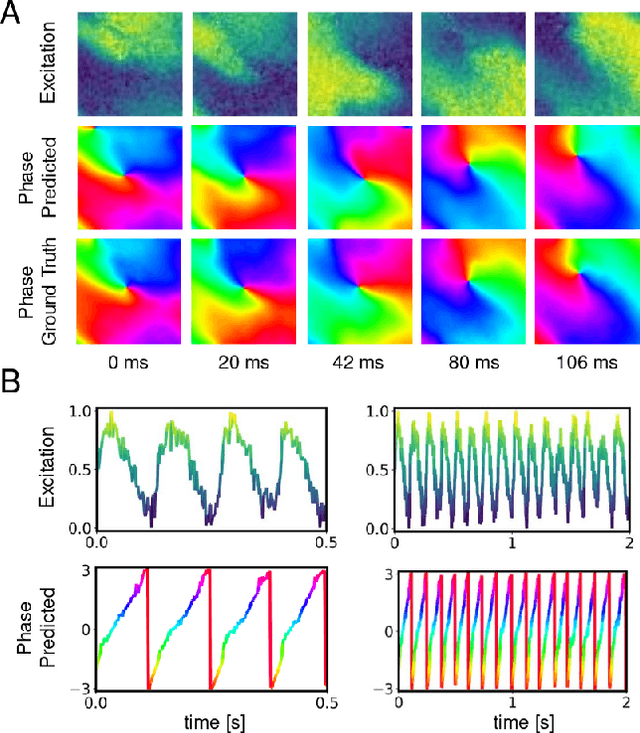
Abstract:The analysis of electrical impulse phenomena in cardiac muscle tissue is important for the diagnosis of heart rhythm disorders and other cardiac pathophysiology. Cardiac mapping techniques acquire numerous local temporal measurements and combine them to visualize the spread of electrophysiological wave phenomena across the heart surface. However, low spatial resolutions, sparse measurement locations, noise and other artifacts make it challenging to accurately visualize spatio-temporal activity. For instance, electro-anatomical catheter mapping is severely limited by the sparsity of the measurements and optical mapping is prone to noise and motion artifacts. In the past, several approaches have been proposed to obtain more reliable maps from noisy or sparse mapping data. Here, we demonstrate that deep learning can be used to compute phase maps and detect phase singularities from both noisy and sparse electrical mapping data with high precision and efficiency. The self-supervised deep learning approach is fundamentally different from classical phase mapping techniques. Rather than encoding a phase signal from time-series data, the network instead learns to directly associate short spatio-temporal sequences of electrical data with phase maps and the positions of phase singularities. Using this method, we were able to accurately compute phase maps and locate rotor cores even from extremely sparse and noisy data, generated from both optical mapping experiments and computer simulations. Neural networks are a promising alternative to conventional phase mapping and rotor core localization methods, that could be used in optical mapping studies in basic cardiovascular research as well as in the clinical setting for the analysis of atrial fibrillation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge