Skylar E. Stolte
BrainFounder: Towards Brain Foundation Models for Neuroimage Analysis
Jun 14, 2024



Abstract:The burgeoning field of brain health research increasingly leverages artificial intelligence (AI) to interpret and analyze neurological data. This study introduces a novel approach towards the creation of medical foundation models by integrating a large-scale multi-modal magnetic resonance imaging (MRI) dataset derived from 41,400 participants in its own. Our method involves a novel two-stage pretraining approach using vision transformers. The first stage is dedicated to encoding anatomical structures in generally healthy brains, identifying key features such as shapes and sizes of different brain regions. The second stage concentrates on spatial information, encompassing aspects like location and the relative positioning of brain structures. We rigorously evaluate our model, BrainFounder, using the Brain Tumor Segmentation (BraTS) challenge and Anatomical Tracings of Lesions After Stroke v2.0 (ATLAS v2.0) datasets. BrainFounder demonstrates a significant performance gain, surpassing the achievements of the previous winning solutions using fully supervised learning. Our findings underscore the impact of scaling up both the complexity of the model and the volume of unlabeled training data derived from generally healthy brains, which enhances the accuracy and predictive capabilities of the model in complex neuroimaging tasks with MRI. The implications of this research provide transformative insights and practical applications in healthcare and make substantial steps towards the creation of foundation models for Medical AI. Our pretrained models and training code can be found at https://github.com/lab-smile/GatorBrain.
DOMINO++: Domain-aware Loss Regularization for Deep Learning Generalizability
Aug 21, 2023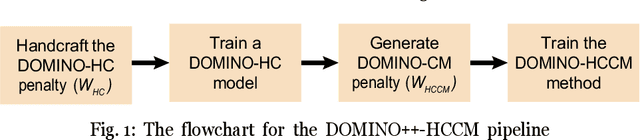
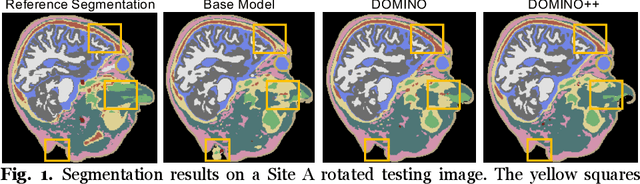
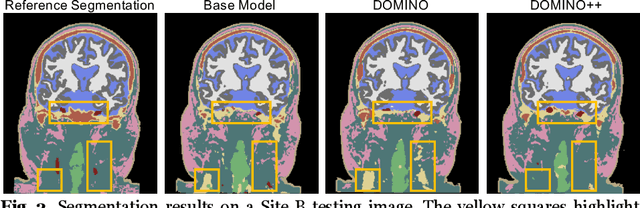

Abstract:Out-of-distribution (OOD) generalization poses a serious challenge for modern deep learning (DL). OOD data consists of test data that is significantly different from the model's training data. DL models that perform well on in-domain test data could struggle on OOD data. Overcoming this discrepancy is essential to the reliable deployment of DL. Proper model calibration decreases the number of spurious connections that are made between model features and class outputs. Hence, calibrated DL can improve OOD generalization by only learning features that are truly indicative of the respective classes. Previous work proposed domain-aware model calibration (DOMINO) to improve DL calibration, but it lacks designs for model generalizability to OOD data. In this work, we propose DOMINO++, a dual-guidance and dynamic domain-aware loss regularization focused on OOD generalizability. DOMINO++ integrates expert-guided and data-guided knowledge in its regularization. Unlike DOMINO which imposed a fixed scaling and regularization rate, DOMINO++ designs a dynamic scaling factor and an adaptive regularization rate. Comprehensive evaluations compare DOMINO++ with DOMINO and the baseline model for head tissue segmentation from magnetic resonance images (MRIs) on OOD data. The OOD data consists of synthetic noisy and rotated datasets, as well as real data using a different MRI scanner from a separate site. DOMINO++'s superior performance demonstrates its potential to improve the trustworthy deployment of DL on real clinical data.
DOMINO: Domain-aware Loss for Deep Learning Calibration
Feb 10, 2023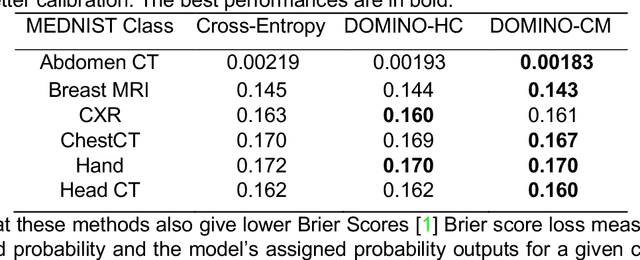
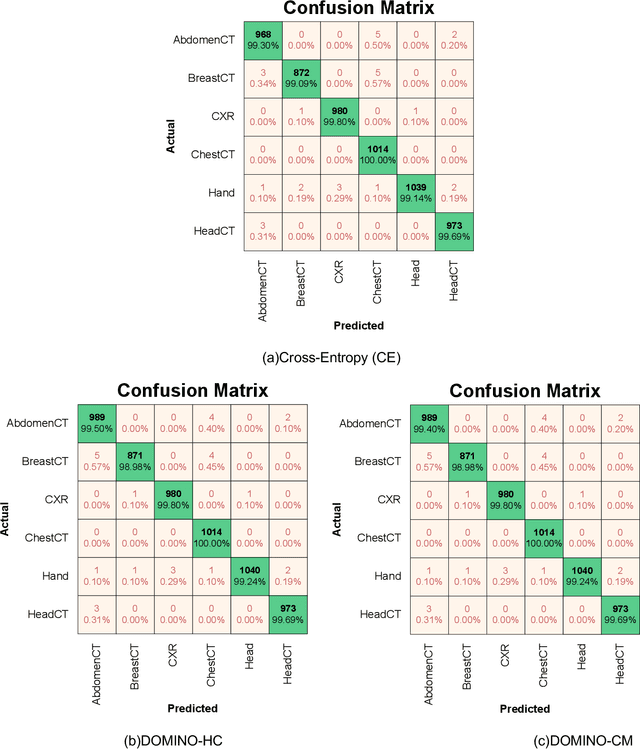
Abstract:Deep learning has achieved the state-of-the-art performance across medical imaging tasks; however, model calibration is often not considered. Uncalibrated models are potentially dangerous in high-risk applications since the user does not know when they will fail. Therefore, this paper proposes a novel domain-aware loss function to calibrate deep learning models. The proposed loss function applies a class-wise penalty based on the similarity between classes within a given target domain. Thus, the approach improves the calibration while also ensuring that the model makes less risky errors even when incorrect. The code for this software is available at https://github.com/lab-smile/DOMINO.
DOMINO: Domain-aware Model Calibration in Medical Image Segmentation
Sep 13, 2022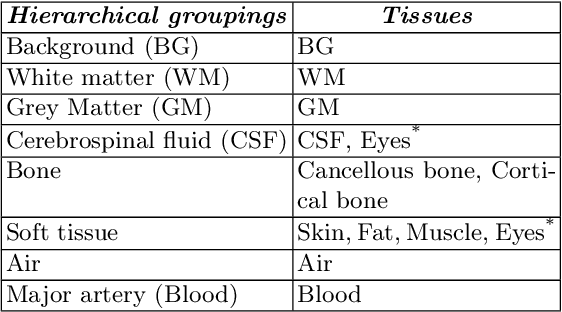
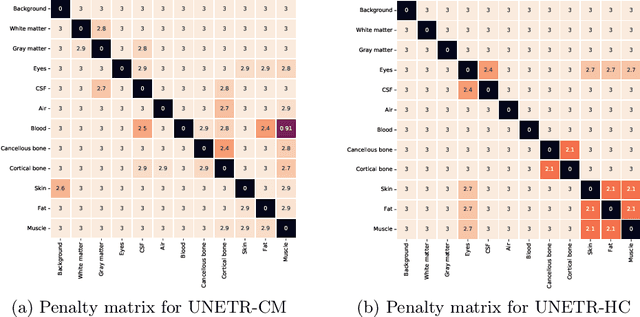
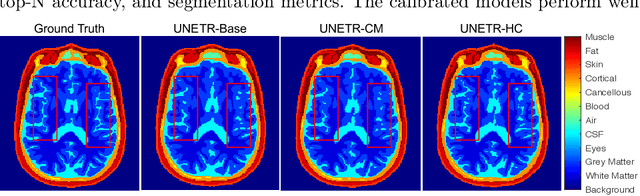
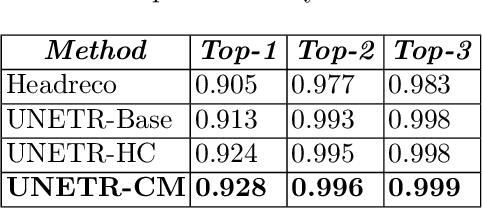
Abstract:Model calibration measures the agreement between the predicted probability estimates and the true correctness likelihood. Proper model calibration is vital for high-risk applications. Unfortunately, modern deep neural networks are poorly calibrated, compromising trustworthiness and reliability. Medical image segmentation particularly suffers from this due to the natural uncertainty of tissue boundaries. This is exasperated by their loss functions, which favor overconfidence in the majority classes. We address these challenges with DOMINO, a domain-aware model calibration method that leverages the semantic confusability and hierarchical similarity between class labels. Our experiments demonstrate that our DOMINO-calibrated deep neural networks outperform non-calibrated models and state-of-the-art morphometric methods in head image segmentation. Our results show that our method can consistently achieve better calibration, higher accuracy, and faster inference times than these methods, especially on rarer classes. This performance is attributed to our domain-aware regularization to inform semantic model calibration. These findings show the importance of semantic ties between class labels in building confidence in deep learning models. The framework has the potential to improve the trustworthiness and reliability of generic medical image segmentation models. The code for this article is available at: https://github.com/lab-smile/DOMINO.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge