Shanmukh Reddy Manne
Diagnostic Quality Assessment of Fundus Photographs: Hierarchical Deep Learning with Clinically Significant Explanations
Feb 18, 2023



Abstract:Fundus photography (FP) remains the primary imaging modality in screening various retinal diseases including age-related macular degeneration, diabetic retinopathy and glaucoma. FP allows the clinician to examine the ocular fundus structures such as the macula, the optic disc (OD) and retinal vessels, whose visibility and clarity in an FP image remain central to ensuring diagnostic accuracy, and hence determine the diagnostic quality (DQ). Images with low DQ, resulting from eye movement, improper illumination and other possible causes, should obviously be recaptured. However, the technician, often unfamiliar with DQ criteria, initiates recapture only based on expert feedback. The process potentially engages the imaging device multiple times for single subject, and wastes the time and effort of the ophthalmologist, the technician and the subject. The burden could be prohibitive in case of teleophthalmology, where obtaining feedback from the remote expert entails additional communication cost and delay. Accordingly, a strong need for automated diagnostic quality assessment (DQA) has been felt, where an image is immediately assigned a DQ category. In response, motivated by the notional continuum of DQ, we propose a hierarchical deep learning (DL) architecture to distinguish between good, usable and unusable categories. On the public EyeQ dataset, we achieve an accuracy of 89.44%, improving upon existing methods. In addition, using gradient based class activation map (Grad-CAM), we generate a visual explanation which agrees with the expert intuition. Future FP cameras equipped with the proposed DQA algorithm will potentially improve the efficacy of the teleophthalmology as well as the traditional system.
Efficient Screening of Diseased Eyes based on Fundus Autofluorescence Images using Support Vector Machine
Apr 17, 2021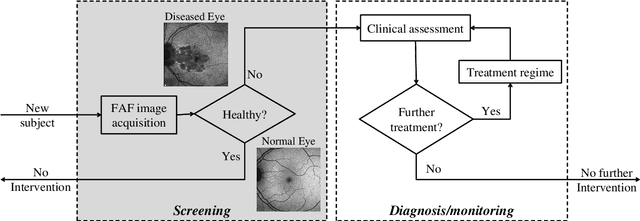


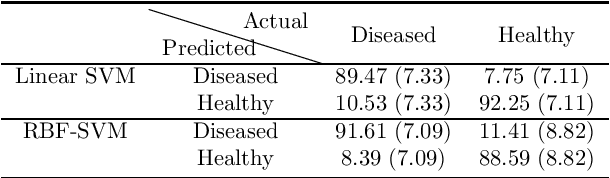
Abstract:A variety of vision ailments are associated with geographic atrophy (GA) in the foveal region of the eye. In current clinical practice, the ophthalmologist manually detects potential presence of such GA based on fundus autofluorescence (FAF) images, and hence diagnoses the disease, when relevant. However, in view of the general scarcity of ophthalmologists relative to the large number of subjects seeking eyecare, especially in remote regions, it becomes imperative to develop methods to direct expert time and effort to medically significant cases. Further, subjects from either disadvantaged background or remote localities, who face considerable economic/physical barrier in consulting trained ophthalmologists, tend to seek medical attention only after being reasonably certain that an adverse condition exists. To serve the interest of both the ophthalmologist and the potential patient, we plan a screening step, where healthy and diseased eyes are algorithmically differentiated with limited input from only optometrists who are relatively more abundant in number. Specifically, an early treatment diabetic retinopathy study (ETDRS) grid is placed by an optometrist on each FAF image, based on which sectoral statistics are automatically collected. Using such statistics as features, healthy and diseased eyes are proposed to be classified by training an algorithm using available medical records. In this connection, we demonstrate the efficacy of support vector machines (SVM). Specifically, we consider SVM with linear as well as radial basis function (RBF) kernel, and observe satisfactory performance of both variants. Among those, we recommend the latter in view of its slight superiority in terms of classification accuracy (90.55% at a standard training-to-test ratio of 80:20), and practical class-conditional costs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge