Sangmin Park
Boundary Estimation from Point Clouds: Algorithms, Guarantees and Applications
Nov 05, 2021
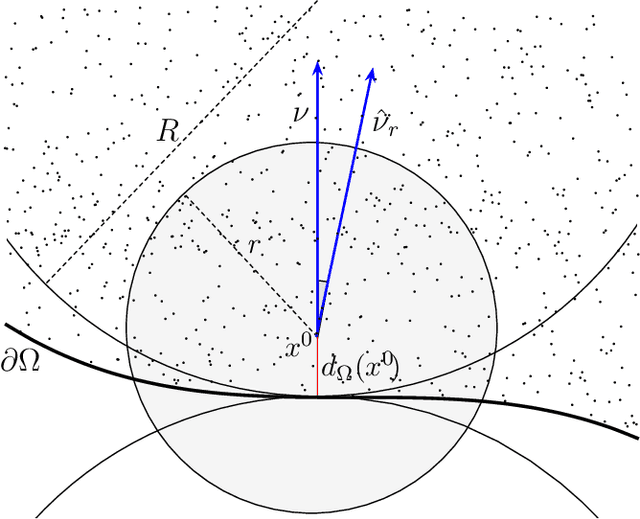
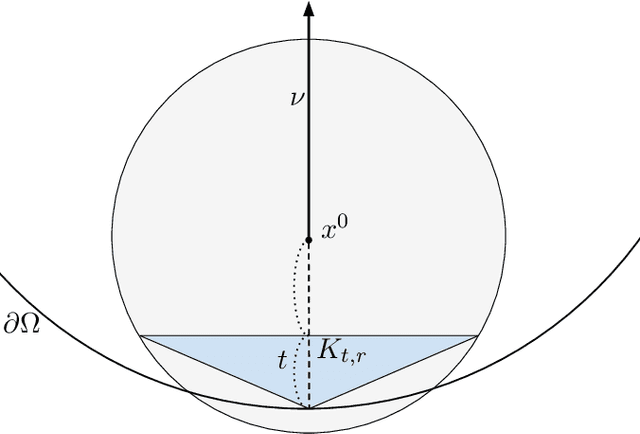
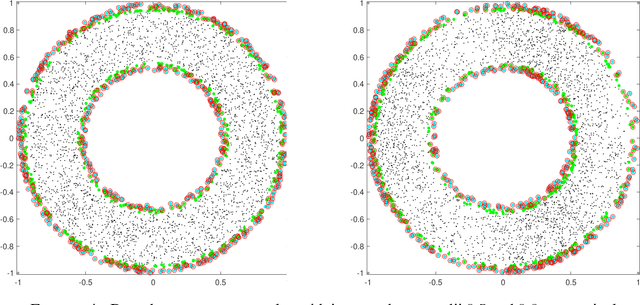
Abstract:We investigate identifying the boundary of a domain from sample points in the domain. We introduce new estimators for the normal vector to the boundary, distance of a point to the boundary, and a test for whether a point lies within a boundary strip. The estimators can be efficiently computed and are more accurate than the ones present in the literature. We provide rigorous error estimates for the estimators. Furthermore we use the detected boundary points to solve boundary-value problems for PDE on point clouds. We prove error estimates for the Laplace and eikonal equations on point clouds. Finally we provide a range of numerical experiments illustrating the performance of our boundary estimators, applications to PDE on point clouds, and tests on image data sets.
Drug-disease Graph: Predicting Adverse Drug Reaction Signals via Graph Neural Network with Clinical Data
Apr 01, 2020

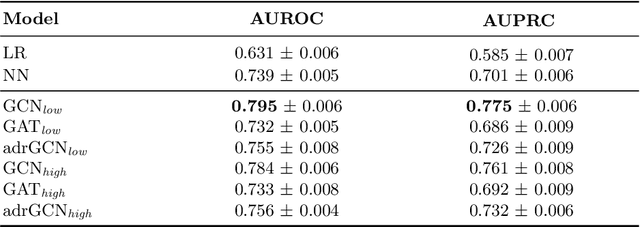

Abstract:Adverse Drug Reaction (ADR) is a significant public health concern world-wide. Numerous graph-based methods have been applied to biomedical graphs for predicting ADRs in pre-marketing phases. ADR detection in post-market surveillance is no less important than pre-marketing assessment, and ADR detection with large-scale clinical data have attracted much attention in recent years. However, there are not many studies considering graph structures from clinical data for detecting an ADR signal, which is a pair of a prescription and a diagnosis that might be a potential ADR. In this study, we develop a novel graph-based framework for ADR signal detection using healthcare claims data. We construct a Drug-disease graph with nodes representing the medical codes. The edges are given as the relationships between two codes, computed using the data. We apply Graph Neural Network to predict ADR signals, using labels from the Side Effect Resource database. The model shows improved AUROC and AUPRC performance of 0.795 and 0.775, compared to other algorithms, showing that it successfully learns node representations expressive of those relationships. Furthermore, our model predicts ADR pairs that do not exist in the established ADR database, showing its capability to supplement the ADR database.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge