Robert Young
Analysis of the MICCAI Brain Tumor Segmentation -- Metastases (BraTS-METS) 2025 Lighthouse Challenge: Brain Metastasis Segmentation on Pre- and Post-treatment MRI
Apr 16, 2025Abstract:Despite continuous advancements in cancer treatment, brain metastatic disease remains a significant complication of primary cancer and is associated with an unfavorable prognosis. One approach for improving diagnosis, management, and outcomes is to implement algorithms based on artificial intelligence for the automated segmentation of both pre- and post-treatment MRI brain images. Such algorithms rely on volumetric criteria for lesion identification and treatment response assessment, which are still not available in clinical practice. Therefore, it is critical to establish tools for rapid volumetric segmentations methods that can be translated to clinical practice and that are trained on high quality annotated data. The BraTS-METS 2025 Lighthouse Challenge aims to address this critical need by establishing inter-rater and intra-rater variability in dataset annotation by generating high quality annotated datasets from four individual instances of segmentation by neuroradiologists while being recorded on video (two instances doing "from scratch" and two instances after AI pre-segmentation). This high-quality annotated dataset will be used for testing phase in 2025 Lighthouse challenge and will be publicly released at the completion of the challenge. The 2025 Lighthouse challenge will also release the 2023 and 2024 segmented datasets that were annotated using an established pipeline of pre-segmentation, student annotation, two neuroradiologists checking, and one neuroradiologist finalizing the process. It builds upon its previous edition by including post-treatment cases in the dataset. Using these high-quality annotated datasets, the 2025 Lighthouse challenge plans to test benchmark algorithms for automated segmentation of pre-and post-treatment brain metastases (BM), trained on diverse and multi-institutional datasets of MRI images obtained from patients with brain metastases.
Establishing the Capabilities of the Murchison Widefield Array as a Passive Radar for the Surveillance of Space
Jun 06, 2022



Abstract:This paper describes the use of the Murchison Widefield Array, a low-frequency radio telescope at a radio-quiet Western Australian site, as a radar receiver forming part of a continent-spanning multistatic radar network for the surveillance of space. This paper details the system geometry employed, the orbit-specific radar signal processing, and the orbit determination algorithms necessary to ensure resident space objects are detected, tracked, and propagated. Finally, the paper includes the results processed after a short collection campaign utilising several FM radio transmitters across the country, up to a maximum baseline distance of over 2500 km. The results demonstrate the Murchison Widefield Array is able to provide widefield and persistent coverage of objects in low Earth orbit.
Direct evaluation of progression or regression of disease burden in brain metastatic disease with Deep Neuroevolution
Mar 24, 2022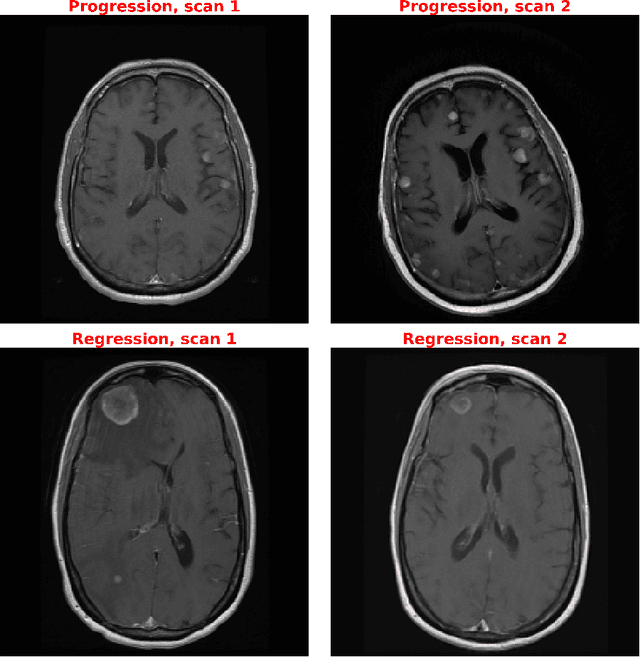
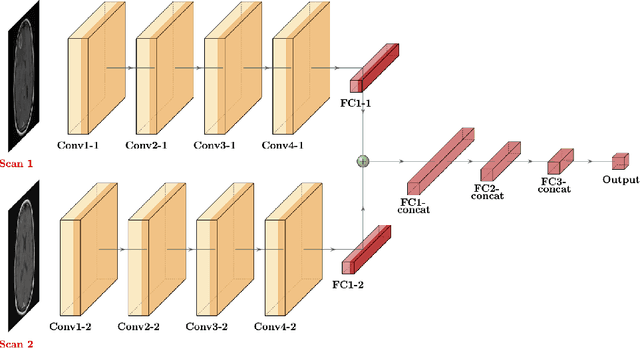
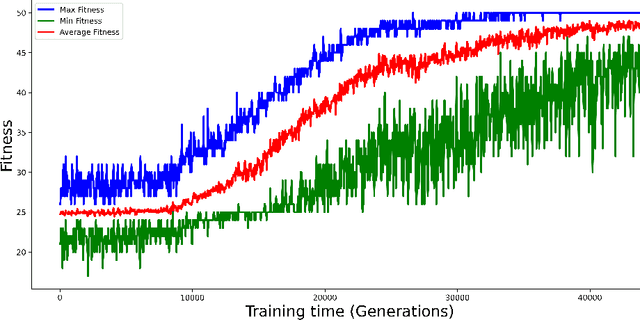
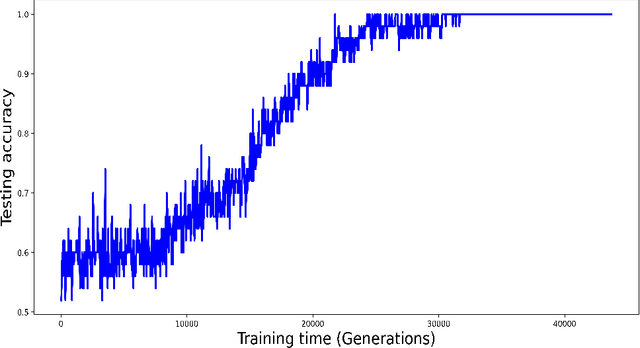
Abstract:Purpose: A core component of advancing cancer treatment research is assessing response to therapy. Doing so by hand, for example as per RECIST or RANO criteria, is tedious, time-consuming, and can miss important tumor response information; most notably, they exclude non-target lesions. We wish to assess change in a holistic fashion that includes all lesions, obtaining simple, informative, and automated assessments of tumor progression or regression. Due to often low patient enrolments in clinical trials, we wish to make response assessments with small training sets. Deep neuroevolution (DNE) can produce radiology artificial intelligence (AI) that performs well on small training sets. Here we use DNE for function approximation that predicts progression versus regression of metastatic brain disease. Methods: We analyzed 50 pairs of MRI contrast-enhanced images as our training set. Half of these pairs, separated in time, qualified as disease progression, while the other 25 images constituted regression. We trained the parameters of a relatively small CNN via mutations that consisted of random CNN weight adjustments and mutation fitness. We then incorporated the best mutations into the next generations CNN, repeating this process for approximately 50,000 generations. We applied the CNNs to our training set, as well as a separate testing set with the same class balance of 25 progression and 25 regression images. Results: DNE achieved monotonic convergence to 100% training set accuracy. DNE also converged monotonically to 100% testing set accuracy. Conclusion: DNE can accurately classify brain-metastatic disease progression versus regression. Future work will extend the input from 2D image slices to full 3D volumes, and include the category of no change. We believe that an approach such as our could ultimately provide a useful adjunct to RANO/RECIST assessment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge