Ping Chai
Myocardial Segmentation of Late Gadolinium Enhanced MR Images by Propagation of Contours from Cine MR Images
May 21, 2022Abstract:Automatic segmentation of myocardium in Late Gadolinium Enhanced (LGE) Cardiac MR (CMR) images is often difficult due to the intensity heterogeneity resulting from accumulation of contrast agent in infarcted areas. In this paper, we propose an automatic segmentation framework that fully utilizes shared information between corresponding cine and LGE images of a same patient. Given myocardial contours in cine CMR images, the proposed framework achieves accurate segmentation of LGE CMR images in a coarse-to-fine manner. Affine registration is first performed between the corresponding cine and LGE image pair, followed by nonrigid registration, and finally local deformation of myocardial contours driven by forces derived from local features of the LGE image. Experimental results on real patient data with expert outlined ground truth show that the proposed framework can generate accurate and reliable results for myocardial segmentation of LGE CMR images.
A Comprehensive 3-D Framework for Automatic Quantification of Late Gadolinium Enhanced Cardiac Magnetic Resonance Images
May 21, 2022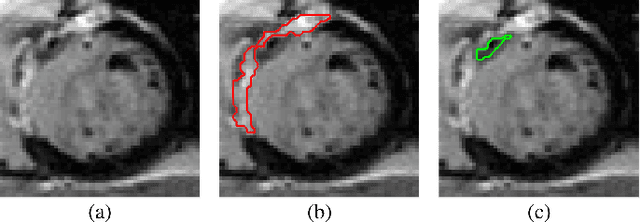
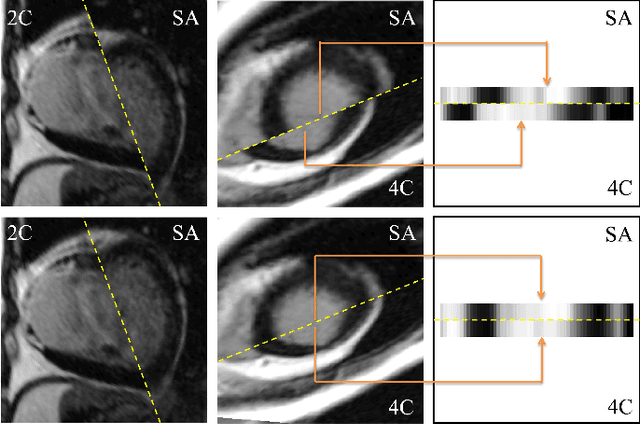
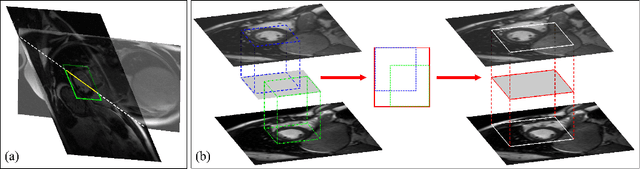
Abstract:Late gadolinium enhanced (LGE) cardiac magnetic resonance (CMR) can directly visualize nonviable myocardium with hyperenhanced intensities with respect to normal myocardium. For heart attack patients, it is crucial to facilitate the decision of appropriate therapy by analyzing and quantifying their LGE CMR images. To achieve accurate quantification, LGE CMR images need to be processed in two steps: segmentation of the myocardium followed by classification of infarcts within the segmented myocardium. However, automatic segmentation is difficult usually due to the intensity heterogeneity of the myocardium and intensity similarity between the infarcts and blood pool. Besides, the slices of an LGE CMR dataset often suffer from spatial and intensity distortions, causing further difficulties in segmentation and classification. In this paper, we present a comprehensive 3-D framework for automatic quantification of LGE CMR images. In this framework, myocardium is segmented with a novel method that deforms coupled endocardial and epicardial meshes and combines information in both short- and long-axis slices, while infarcts are classified with a graph-cut algorithm incorporating intensity and spatial information. Moreover, both spatial and intensity distortions are effectively corrected with specially designed countermeasures. Experiments with 20 sets of real patient data show visually good segmentation and classification results that are quantitatively in strong agreement with those manually obtained by experts.
Three-Dimensional Segmentation of the Left Ventricle in Late Gadolinium Enhanced MR Images of Chronic Infarction Combining Long- and Short-Axis Information
May 21, 2022
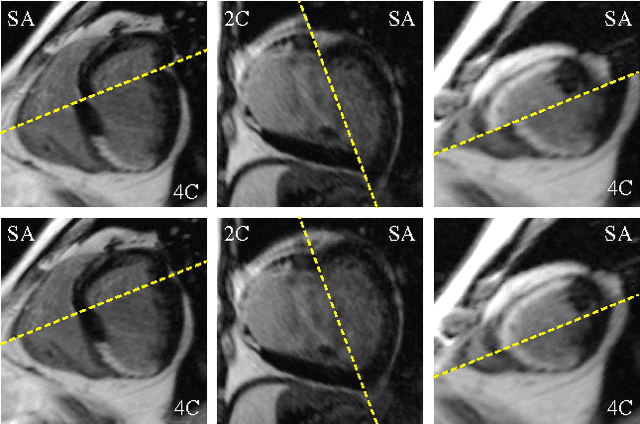
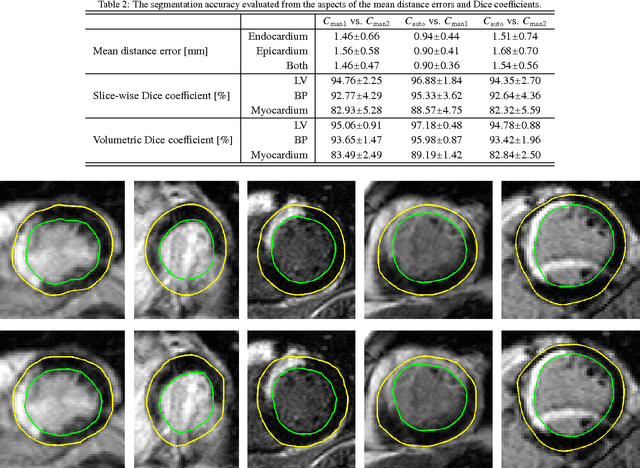
Abstract:Automatic segmentation of the left ventricle (LV) in late gadolinium enhanced (LGE) cardiac MR (CMR) images is difficult due to the intensity heterogeneity arising from accumulation of contrast agent in infarcted myocardium. In this paper, we present a comprehensive framework for automatic 3D segmentation of the LV in LGE CMR images. Given myocardial contours in cine images as a priori knowledge, the framework initially propagates the a priori segmentation from cine to LGE images via 2D translational registration. Two meshes representing respectively endocardial and epicardial surfaces are then constructed with the propagated contours. After construction, the two meshes are deformed towards the myocardial edge points detected in both short-axis and long-axis LGE images in a unified 3D coordinate system. Taking into account the intensity characteristics of the LV in LGE images, we propose a novel parametric model of the LV for consistent myocardial edge points detection regardless of pathological status of the myocardium (infarcted or healthy) and of the type of the LGE images (short-axis or long-axis). We have evaluated the proposed framework with 21 sets of real patient and 4 sets of simulated phantom data. Both distance- and region-based performance metrics confirm the observation that the framework can generate accurate and reliable results for myocardial segmentation of LGE images. We have also tested the robustness of the framework with respect to varied a priori segmentation in both practical and simulated settings. Experimental results show that the proposed framework can greatly compensate variations in the given a priori knowledge and consistently produce accurate segmentations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge