Patrik Bachtiger
In-ear ECG Signal Enhancement with Denoising Convolutional Autoencoders
Aug 27, 2024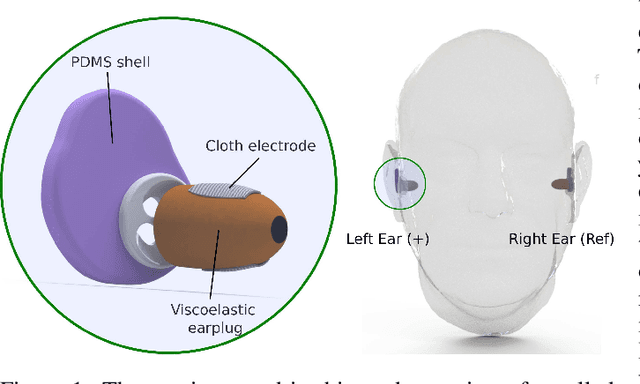
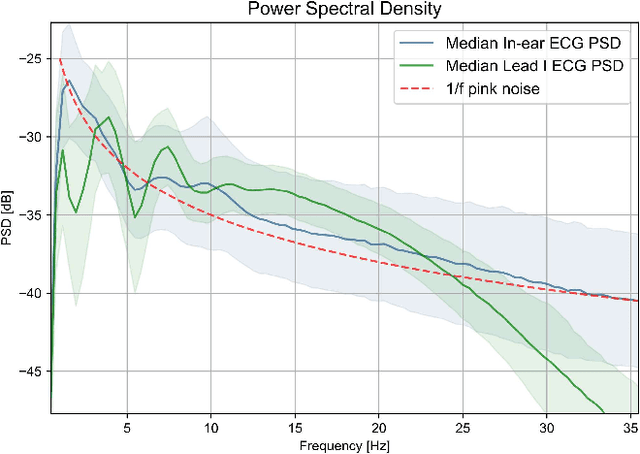
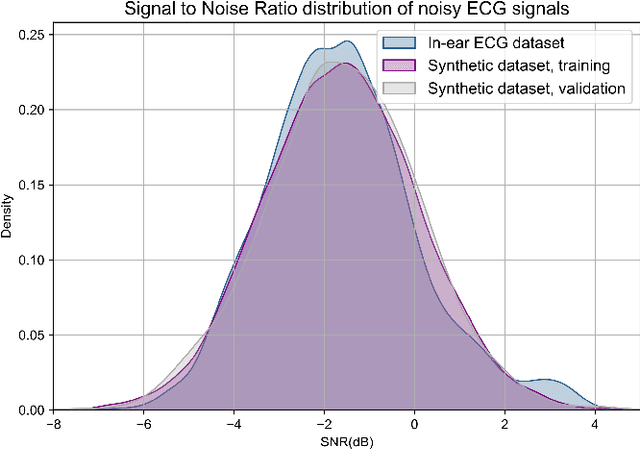
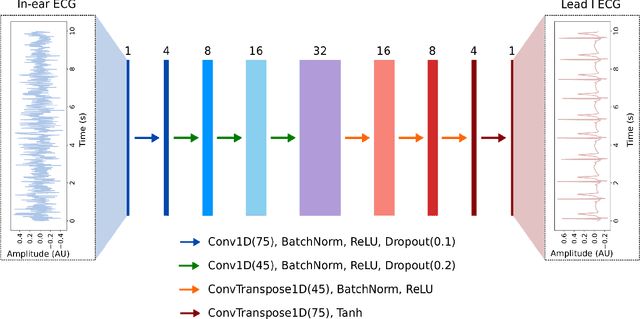
Abstract:The cardiac dipole has been shown to propagate to the ears, now a common site for consumer wearable electronics, enabling the recording of electrocardiogram (ECG) signals. However, in-ear ECG recordings often suffer from significant noise due to their small amplitude and the presence of other physiological signals, such as electroencephalogram (EEG), which complicates the extraction of cardiovascular features. This study addresses this issue by developing a denoising convolutional autoencoder (DCAE) to enhance ECG information from in-ear recordings, producing cleaner ECG outputs. The model is evaluated using a dataset of in-ear ECGs and corresponding clean Lead I ECGs from 45 healthy participants. The results demonstrate a substantial improvement in signal-to-noise ratio (SNR), with a median increase of 5.9 dB. Additionally, the model significantly improved heart rate estimation accuracy, reducing the mean absolute error by almost 70% and increasing R-peak detection precision to a median value of 90%. We also trained and validated the model using a synthetic dataset, generated from real ECG signals, including abnormal cardiac morphologies, corrupted by pink noise. The results obtained show effective removal of noise sources with clinically plausible waveform reconstruction ability.
A Framework for Predicting Impactability of Healthcare Interventions Using Machine Learning Methods, Administrative Claims, Sociodemographic and App Generated Data
May 15, 2019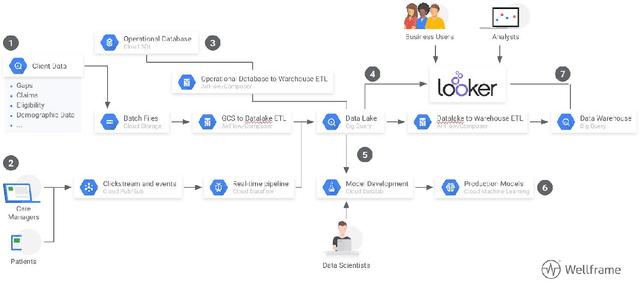
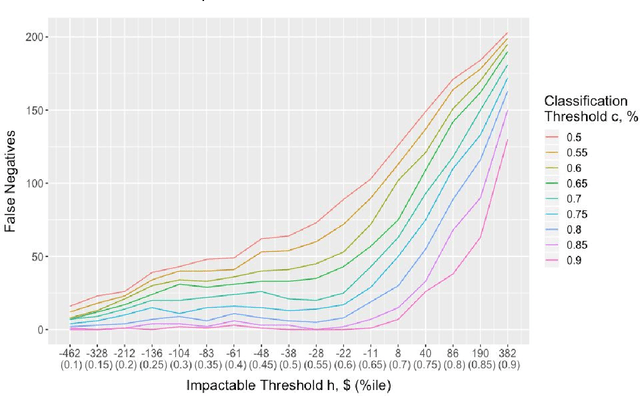
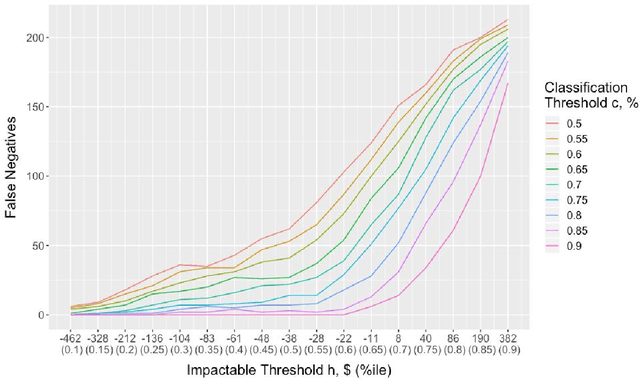
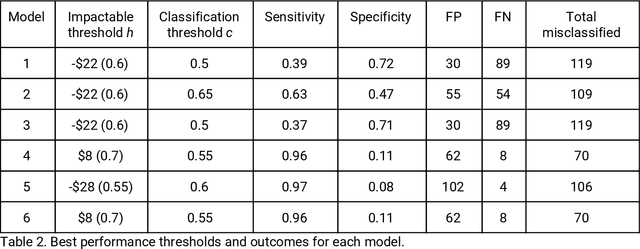
Abstract:It is not clear how to target patients who are most likely to benefit from digital care management programs ex-ante, a shortcoming of current risk score based approaches. This study focuses on defining impactability by identifying those patients most likely to benefit from technology enabled care management, delivered through a digital health platform, including a mobile app and clinician web dashboard. Anonymized insurance claims data were used from a commercially insured population across several U.S. states and combined with inferred sociodemographic data and data derived from the patient-held mobile application itself. Our approach involves the creation of two models and the comparative analysis of the methodologies and performances therein. We first train a cost prediction model to calculate the differences in predicted (without intervention) versus actual (with onboarding onto digital health platform) healthcare expenditure for patients (N = 1,242). This enables the classification of impactability if differences in predicted versus actual costs meet a predetermined threshold. A random forest machine learning model was then trained to accurately categorize new patients as impactable versus not impactable, reaching an overall accuracy of 71.9%. We then modify these parameters through grid search to define the parameters that deliver optimal performance. A roadmap is proposed to iteratively improve the performance of the model. As the number of newly onboarded patients and length of use continues to increase, the accuracy of predicting impactability will improve commensurately as more advanced machine learning techniques such as deep learning become relevant. This approach is generalizable to analyzing the impactability of any intervention and is a key component of realising closed loop feedback systems for continuous improvement in healthcare.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge