Natasha Mulligan
Evaluating the Predictive Features of Person-Centric Knowledge Graph Embeddings: Unfolding Ablation Studies
Aug 29, 2024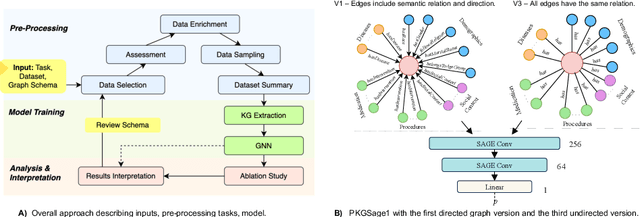
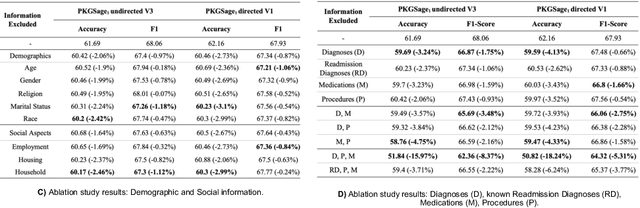
Abstract:Developing novel predictive models with complex biomedical information is challenging due to various idiosyncrasies related to heterogeneity, standardization or sparseness of the data. We previously introduced a person-centric ontology to organize information about individual patients, and a representation learning framework to extract person-centric knowledge graphs (PKGs) and to train Graph Neural Networks (GNNs). In this paper, we propose a systematic approach to examine the results of GNN models trained with both structured and unstructured information from the MIMIC-III dataset. Through ablation studies on different clinical, demographic, and social data, we show the robustness of this approach in identifying predictive features in PKGs for the task of readmission prediction.
* Published in the 34th Medical Informatics Europe Conference
Representation Learning for Person or Entity-centric Knowledge Graphs: An Application in Healthcare
May 10, 2023



Abstract:Knowledge graphs (KGs) are a popular way to organise information based on ontologies or schemas and have been used across a variety of scenarios from search to recommendation. Despite advances in KGs, representing knowledge remains a non-trivial task across industries and it is especially challenging in the biomedical and healthcare domains due to complex interdependent relations between entities, heterogeneity, lack of standardization, and sparseness of data. KGs are used to discover diagnoses or prioritize genes relevant to disease, but they often rely on schemas that are not centred around a node or entity of interest, such as a person. Entity-centric KGs are relatively unexplored but hold promise in representing important facets connected to a central node and unlocking downstream tasks beyond graph traversal and reasoning, such as generating graph embeddings and training graph neural networks for a wide range of predictive tasks. This paper presents an end-to-end representation learning framework to extract entity-centric KGs from structured and unstructured data. We introduce a star-shaped ontology to represent the multiple facets of a person and use it to guide KG creation. Compact representations of the graphs are created leveraging graph neural networks and experiments are conducted using different levels of heterogeneity or explicitness. A readmission prediction task is used to evaluate the results of the proposed framework, showing a stable system, robust to missing data, that outperforms a range of baseline machine learning classifiers. We highlight that this approach has several potential applications across domains and is open-sourced. Lastly, we discuss lessons learned, challenges, and next steps for the adoption of the framework in practice.
Deep EHR Spotlight: a Framework and Mechanism to Highlight Events in Electronic Health Records for Explainable Predictions
Mar 25, 2021Abstract:The wide adoption of Electronic Health Records (EHR) has resulted in large amounts of clinical data becoming available, which promises to support service delivery and advance clinical and informatics research. Deep learning techniques have demonstrated performance in predictive analytic tasks using EHRs yet they typically lack model result transparency or explainability functionalities and require cumbersome pre-processing tasks. Moreover, EHRs contain heterogeneous and multi-modal data points such as text, numbers and time series which further hinder visualisation and interpretability. This paper proposes a deep learning framework to: 1) encode patient pathways from EHRs into images, 2) highlight important events within pathway images, and 3) enable more complex predictions with additional intelligibility. The proposed method relies on a deep attention mechanism for visualisation of the predictions and allows predicting multiple sequential outcomes.
* AMIA 2021 Virtual Informatics Summit
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge